Researchers at the RIKEN BioResource Research Center, Kyoto, Japan, have reported that retinal organoids, derived from patients’ induced pluripotent stem cells (iPSCs) with a EYS (eye shut homolog gene)-associated retinal dystrophy (EYS-RD), have provided valuable insight on the phototoxic effects of the retinal pathology. Photoreceptor cells of RD organoids, derived from patients, showed that certain proteins handling phototoxicity were not in the outer segments. In addition, photoreceptor cells in RD organoids appeared to be vulnerable to light stimuli and especially to blue light. The researchers commented that, “mislocalisation of GRK7 (G protein–coupled receptor kinase 7), which was also observed in eys-knockout zebrafish, was reversed by delivering control EYS into photoreceptor cells of RD organoids. These findings suggest that avoiding phototoxicity would be a potential therapeutic approach for EYS-RD”.
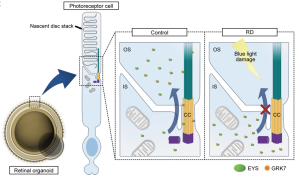
Fig. 1: Schematic representation of putative mechanism of EYS-RD. EYS interacts with GRK7 and transports it from the IS to the OS. In control photoreceptor cells, EYS can localize at the CC (connecting cilia) and OS (outer segments), whereas in RD, EYS with GRK7 cannot distribute to these regions. Light-induced damage via excessive reactive oxygen species production is caused by the mislocalization of mutant EYS with GRK7. [JCI Insight 2024;9(8):e174179 https://doi.org/10.1172/jci.insight.174179, https://creativecommons.org/licenses/by/4.0/]
The literature previously outlined that the eyes shut homolog (EYS) gene was first identified as an autosomal recessive causative gene for retinitis pigmentosa (RP) in 2008, reporting one of the most frequent RP causes with a prevalence of ~5%–30% worldwide. The gene is located on chromosome 6p12, comprising 2 Mb of genomic DNA across 44 exons, and encodes a 3,165–amino acid protein, is one of the largest molecules expressed in the human eye. According to the current study, the Japanese researchers were aimed to investigate the pathogenesis of EYS-RD generating retinal organoids from patient-derived iPSCs highlighting that, “this is the first report to our knowledge about human iPSCs being used to study the molecular mechanism of EYS function and its pathogenesis”. Human EYS was localized at the connecting cilia (CC) and OS (outer segments) in photoreceptor cells, “whereas mutant truncated EYS did not distribute to these regions. We found that EYS plays a role in the trafficking of an OS protein, G protein–coupled receptor kinase 7 (GRK7), which is evolutionarily conserved along with EYS and involved in photoresponse recovery”.
Following the research, the study showed that GRK7 dysfunction in the RD organoids caused by mutant EYS led to the overresponse to light stimuli and resulted in cell death via ROS (reactive oxygen species) generation. Their data suggested that light-induced cell death in RD organoids was especially caused by short-wavelength light. The results showed that blue light appeared to cause the greatest amount of photoreceptor cell death and that intense-energy light may highly drive the phototransduction cascade in light-sensitive RD organoids. Accordingly, the researchers commented that, “since the illuminance of blue light tends to be underestimated by human eyes, cutting harmful short-wavelength light would be beneficial for patients with EYS-RD by using tinted glasses or other devices”. In summary, the RIKEN team’s work suggest that the outcomes, “would be a potential therapeutic approach for patients with EYS-RD to restrict the exposure to high-energy short-wavelength light”.