Researchers at the Department of Genetics, and the Department of Ophthalmology, Harvard Medical School, Boston, have reported a novel gene-agnostic strategy treating retinitis pigmentosa (RP), aimed at promoting lactate uptake from the blood and encouraging the passage of glucose from the RPE to cone cells. Given that RP is associated ~100 genes, and a larger number of mutations, a gene therapy approach for each variant is unfeasible, in terms of phenotypes, time and economics. The Harvard team instead have proposed targeting a secondary effect of RP pathology – a loss of cone cells, which lead to the deterioration of daylight colour and vision. The pre-clinical models of RP have now shown a proof-of-concept, providing prolonged survival and function of cones “revealing a possible gene-agnostic therapy for preserving vision in RP”, published in the Proceedings of the National Academy Sciences (Apr. 3rd, 2025, Vol. 144, No. 14).
Given the use of cone vision required for many activities in daily life, extending colour vision is of significant importance for RP patients. Given that rod and cone photoreceptors are extremely metabolically active, these cells use glucose via aerobic glycolysis to generate significant quantities of lactate. Without vascularization in the photoreceptor layer, the passage of glucose from the choroid, is to be mediated via the retinal pigment epithelium (RPE). In their current modelling, when the rod photoreceptor cell population decreases, as when RP occurs, there is a reduction of level of lactate available to the RPE. The Harvard gene-agnostic strategy was “aimed to encourage the RPE to take up lactate from the blood and relinquish glucose to the surviving cones, by expressing a high-affinity lactate transporter, MCT2 (monocarboxylate transporter 2), specifically within RPE cells using an adeno-associated viral (AAV) vector”. In research team showed that AAV vector-mediated delivery of MCT2 into pre-clinical models of RP increased cone cell survival and function across four different disease-causing mutations.
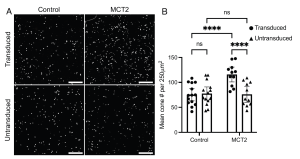
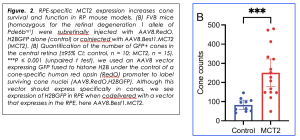
Figure 1. RPE-specific monocarboxylate transporter 2 (MCT2) expression increases cone survival. S334ter pre-clinical models were subretinally injected with red fluorescent beads alone (control) or co-injected with AAV8.Best1.MCT2 (MCT2). (A) Representative 500 μm2 images taken from the mid-periphery of transduced and untransduced regions from control and MCT2 retinae. (Scale bar, 100 μm.) (B) Absolute cone counts averaged across four 250 μm2 regions within the transduced and untransduced regions of control and MCT2 samples (±95% CI; control, n = 14; MCT2, n = 11). ****P ≤ 0.0001 (two-way repeated measures ANOVA with Šídák’s multiple comparison test). The density of cones in a wild-type Sprague-Dawley rat at approximately P84 is 407.5 ± 47.07 per 250 μm2 (n = 8; average of both eyes). [This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/), authored by Chandler et al., entitled, “RPE-specific MCT2 expression promotes cone survival in models of retinitis pigmentosa”, published in PNAS, Apr. 3rd, 2025, Vol. 144, No. 14, https://doi.org/10.1073/pnas.2421978122.]
The strategy was aimed to promote lactate uptake from the adjacent choroidal vasculature and then encourage the RPE to release more glucose for uptake by cones. Their results prolonged survival and function of cones in the pre-clinical models, providing a proof-of-concept. However, the data also showed that the effect diminished with time and therefore further research may be required to extend these outcomes. Regardless, given there is no current cure for such RP patients, this new study may provide hope to further tackle these secondary effects of RP. Commenting on the work, the researchers said that, “it is likely that other mechanisms also contribute to cone degeneration, including inflammation and oxidative damage. To achieve sustained rescue, each of these may need to be addressed in a combinatorial manner. Nevertheless, these data support the hypothesis that the metabolic status of the RPE impact cone survival and reveals a gene-agnostic therapy for prolonging vision in RP”.