Researchers based at the Department of Biomedical Engineering, University of Houston, Texas and the Department of Ophthalmology, Duke University Medical Centre, Durham, North Carolina, have reported a Nature Communications article (Nat. Comm. 2024,15:4756) presenting data on the reduction of rhodopsin protein levels for the purpose of ameliorating the impact of mutations on peripherin. While seemingly counter-intuitive, the strategy is aimed to recalibrate the ratio of rhodopsin (RHO):peripherin-2/rds (PRPH2), thereby improving functional and anatomical recovery. According to the researchers, there are no approved treatments or ongoing clinical trials specifically for PRPH2 associated diseases however, the current strategy may provide an attractive strategy to reduce rhodopsin protein levels that supports vision biochemistry and minimise periphrin-2/rds pathology.
Perpherin-2/RDS protein is a ~39 kDa integral membrane glycoprotein that is part of the tetraspanin superfamily and mutations in peripherin-2 have been associated with retinitis pigmentosa and macular dystrophy and mutations in peripherin-2/rds (PRPH2) have identified ~200 pathogenic variants. In rod photoreceptors, PRPH2 is located in the outer segment (OS) disc rim region and forms homo-tetramers as well as hetero-tetramers with its homolog, rod outer segment membrane protein 1 (ROM1). The tetramers can then assemble octamers and higher order oligomers, playing a crucial role in OS disc rim formation and a minimum threshold PRPH2 protein levels (~ 80% of wild type) are known to be essential for OS disc morphogenesis and maintenance. Researchers have shown that the ratio of PRPH2 to the rod-specific protein rhodopsin (RHO) plays a crucial role in morphogenesis of rod OS discs, and models with a decreased ratio appear to display properly oriented rod outer segments (ROSs).
In the current report, researchers showed that reducing one allele of Rho in heterozygous models improves photoreceptor ultrastructure and physiological function (using a knock-in mouse model, Prph2Y141C/+. In addition, researchers demonstrate the translational applicability of this strategy, using “a previously characterized antisense oligonucleotide (ASO) mRho ASO1 shown to effectively reduce Rho transcript levels following intravitreal administration”. Their paper showed that their results provided, “improved retinal function, OS ultrastructure, and delayed photoreceptor degeneration” and “these findings suggest that reducing RHO levels is a potential therapeutic strategy to ameliorate the disease phenotype in patients with PRPH2-associated inherited retinal disorders”.
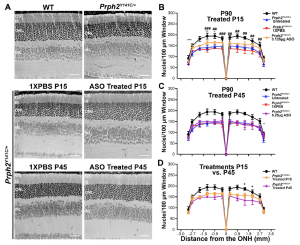
Figure 1. Early-stage treatment with mRhoASO1 successfully delays ONL thinning in Prph2Y141C/+ mice. A Representative images of retinal sections stained with H&E at P90. B–D Nuclear counts from100 μm-windows at every 500 μm distance from the optic nerve and across the superior-inferior plane of retinal sections collected from P90 un-injected, 1X PBS injected as a control and mRho ASO1 injected animals following (B) early (P15) or (C) late-stage (P45) therapeutic intervention. B, C WT and Prph2Y141C/+ controls were added for comparison. B, C∗P<0.05, ∗∗P<0.01, ∗∗∗P < 0.001 by two-way ANOVA (P15 Injected: Interaction P<0.0001, Row Factor P < 0.0001, and Column Factor P < 0.0001. P45 Injected: Interaction P<0.0001, Row Factor P < 0.0001, and Column Factor P < 0.0001. P15 vs. P45 Injected: Interaction P<0.0001, Row Factor P < 0.0001, and Column Factor P < 0.0001.) with Tukey’s post-hoc comparison. *denotes comparisons between Prph2Y141C/+ and (B) Prph2Y141C/+ 3.125 μg ASO or (C) Prph2Y141C/+ 6.25 μg ASO. #denotes comparisons between (B) Prph2Y141C/+ 3.125 μg ASO and P15 injected Prph2Y141C/+ 1X PBS. D Plotted are mean ± SD values from(B) and(C) fo rWT, Prph2Y141C/+ P15 Injected 3.125 μg ASO, and P45 injected 6.25 μg ASOPrph2Y141C/+ for ease of comparison. + denotes comparisons P15 injected and P45 injected. N = 3 animals for all genotypes and experimental conditions. Scale bar corresponds to 50 μm. (Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/ licenses/by/4.0/.)
While these experimental treatments are in an early stage, the researchers commented that, “these findings further underscore the importance of maintaining proper RHO:PRPH2 ratios and choosing the appropriate time point for intervention to effectively prevent photoreceptor death using the reduction of RHO as a strategy”. In addition, researchers concluded that, “future investigations will focus on expanding the application of this strategy to include additional Prph2mutations, exploring the long term effects of Rho knockdown in healthy controls, and assessing the feasibility of repeated dosing in long-term Prph2 mutants“.