A team of Korean researchers have published a study using an AI-model of optical coherence tomography (OCT) image data to predict AMD treatment outcomes following anti-VEGF injections. The report proposes a potential AI tool for personalised ophthalmic care and the researchers’ data showed that their AI model has surpassed the predictive performance of ophthalmologists. The research was conducted at the Department of Ophthalmology, Seoul National University College of Medicine, Seoul, and the Department of Applied Artificial Intelligence, Sungkyunkwan University, Seoul, and their paper was published in the prestigious Nature journal Scientific Reports, (2024)14:28253.
The researchers outlined that while OCT is one of the most important tools for evaluating the anatomical condition of the macula, it may not be able to precisely predict the functional improvement in visual acuity (VA). The dynamic environment in the active stage of AMD has several components, including the presence of new or persistent intraretinal fluid (IRF), new or persistent subretinal fluid (SRF), enlargement of retinal pigment epithelial detachment (PED), or presence of subretinal hyperreflective material (SHRM). In addition, functional improvement varies depending on the location of the fluid, and IRF is highly relevant to this in terms of poor visual prognosis. To tackle these challenges, artificial intelligence (AI) is aimed to acquire IRF, SRF and PED volumes with OCT imaging using automation, and thereafter, predict the degree of vision improvement following anti-VEGF injection using each computed volume of the fluid. A machine-learning algorithm is used to assess the treatment burden associated with AMD and a model that predicts the OCT status following anti-VEGF injection, by learning pre-treatment, uses a generative adversarial network (GAN). The researchers indicate that implementation of AI could be useful in informing patients about their conditions after treatment. The researchers stated that, “the predictive performance of the model could be improved using OCT images after the first and second treatments, as well as pre-injection images. In addition, the performance of the ophthalmologists was investigated and compared with the performance of the model”.
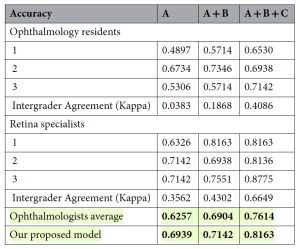
Following their work, the team collated 2,068 OCT images from 517 patients with neovascular AMD, with a mean age of 71.4 ± 9.0 years (range, 65–78; median 72). The performance model metrics evaluated the OCT images for: before injection, after the first injection, and after the second injection. The AI model results outperformed both resident and retinal specialist groups, demonstrating approximately 7%, 2%, and 5% higher accuracy, than the average ophthalmologist performance. The researchers commented that, “this experiment highlights the robustness of the design, as increased image availability led to better human performance, yet the AI model maintained a superior level of accuracy, underscoring its potential clinical value.”
Table 1. Model performance comparison between the ophthalmologists. The patients’ spectral-domain optical coherence tomography images captured before injection, after the first injection, and after the second injection are denoted as A, B, and C, respectively. Both ophthalmologists and our proposed model used these images as inputs to predict the results. Fleiss’ kappa scores were used to calculate intergrader agreement, revealing that agreement improved as more OCT images were provided. The AI model consistently demonstrated higher predictive accuracy compared to both resident and specialist groups across all conditions. Additionally, the p-value confirmed that the observed improvements in agreement were statistically significant, with a p-value of 0.05 or lower for intergrader agreements exceeding 0.1. Significant values are in [bold].[Open Access: this article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, authored by Jeong Mo Han et al, entitled: Anti-VEGF treatment outcome prediction based on optical coherence tomography images in neovascular age-related macular degeneration using a deep neural network. Scientific Reports (2024)14:28253 https://doi.org/10.1038/s41598-024-79034-6y].
The research team commented that this was the “first study to attempt to compare the prediction results of AI with those of ophthalmologists and retinal specialists regarding whether the nAMD status would become inactive after treatment, as well as predict the results after three loading injections using not only images before treatment but also images taken during the treatment process.” While the researchers cautioned that while there were a number of limitations (small number of patients, the type of anti-VEGF agent, the limited demographic population and the measurement of VA), they concluded that their “model demonstrated a higher mean performance than ophthalmologists, and the model’s treatment prediction performance further improved with additional OCT images during the loading phase.”