A consortium of researchers from Australia, New Zealand, USA, UK and Switzerland, have published paper for a new treatment outcome and natural history registry for inherited retinal disease (IRD), under the auspices of “THE FIGHT INHERITED RETINAL BLINDNESS! PROJECT”. The study proposed a new disease registry to track the natural history and outcomes of approved gene therapy in patients with inherited retinal diseases, aimed to collect valuable real-world data and made available online. The Fight Inherited Retinal Blindness! project provides an organized, web-based system that “uses observational study methods to collect uniform data from patients with inherited retinal disease to track natural history and (uniquely) treatment outcomes. It is free to users who have control over their data”.
The Fight Inherited Retinal Blindness! (FIRB!) Registry is an expanded registry building on previous modules supported by the Save Sight Registries, a non-profit organisation, also supported with the University of Sydney, Australia. A committee of six members was overseen the construction of the Fight Inherited Retinal Blindness! Module with a further 11 experts all of whom have a clinical and/or research interest in IRDs. The web-based Fight Inherited Retinal Blindness! registry records baseline demographic, clinical, and genetic data together with follow-up data. The Human Phenotype Ontology and Monarch Disease Ontology nomenclature were incorporated within the Fight Inherited Retinal Blindness! architecture to standardize nomenclature (Mondo: unifying diseases for the world, by the world. medRxiv 2022: 1–12). The registry has developed software that can assign individual diagnoses to one of seven broad phenotypic groups, with minimum datasets dependent on the broad phenotypic group. New patient entries can be completed in 5 minutes, and follow-up data can be entered in 2 minutes.
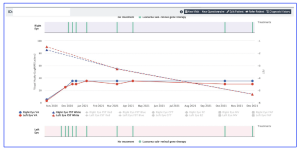
Figure 1. Screengrab of a data entry/presentation page for a patient with IRD secondary to biallelic RPE65 mutations after retinal gene therapy with voretigene neparvovec. Pertinent clinical data (e.g., best-corrected visual acuity, FST results) can be plotted against time as a means of easily tracking disease progression and treatment effects. [The research work is licensed under the terms of the Creative Commons Attribution-Non Commercial-No Derivatives License 4.0 (CCBY-NC-ND), cited by Simunovic et al., entitled by: “THE FIGHT INHERITED RETINAL BLINDNESS! PROJECT A New Treatment Outcome and Natural History Registry for Inherited Retinal Disease”, Retina, 2025; 45:286–295 ].
According to the researchers, The Fight Inherited Retinal Blindness Registry! was conceived for two purposes: “first, to track the natural history of IRDs, and second, to monitor real-world outcomes in patients receiving emerging treatments, such as gene therapy”. The FIRB! registry provides a rapid means of recording outcomes from treatments, for example, following Luxturna gene therapy (voretigene neparvovec). The outcome measures are those “recommended by both regulatory authorities and professional bodies internationally, making the registry a convenient and rapid means of recording outcomes to assess real world outcomes and for drug monitoring by national authorities”. In addition, FIRB! provides a means of “tracking adverse or unanticipated events—such as the development of chorioretinal atrophy—which has only been reported post regulatory approval in a significant minority of patients undergoing voretigene neparvovec gene therapy”.