Researchers at the Ottawa Hospital Research Institute, Ontario, Canada have reported a systematic review and meta-analysis on the safety and efficacy of adeno-associated viral (AAV) gene therapy treatments for patients with retinal gene therapy. The retinal degenerations that were evaluated included Leber congenital amaurosis (LCA), choroideremia, Leber hereditary optic neuropathy (LHON), age-related macular degeneration (AMD), retinitis pigmentosa (RP), X-linked retinoschisis, and achromatopsia. While the differences on the disease type, treatments and methodologies were significantly disparate, the researchers concluded that the surgical procedure to deliver AAV was associated with some risk. The small size on the individual studies and the payload of the different genes being used may require further analyses and follow-up.
The systematic review collected a total of 3,548 citations, from which 80 articles, representing 28 clinical trials and 5 post-market studies. A total of 595 patients were enrolled across the review – sixteen of the 28 registered clinical trials (63%) involved dose escalation studies, the majority of trials (23 of 28) were early phase (I/II) clinical trials, and 20 had safety as the primary outcome. The targeted gene/proteins evaluated was RPE65 in 7 trials and 5 post-market surveillance studies, ND4 in 7 studies, CHM/REP1 in 5 studies, sFLT-1 in 2 studies, CNGA3 in 2 studies, and one trial each for GUCY2D, MERTK, RPGR, RS1, and ChrimsonR. Overall, AAV therapy vectors were associated with a cumulative incidence of at least one SAE (serious adverse event) of 8% (95% confidence intervals [CIs] of 5% to 12%). SAEs were often associated with the surgical procedure rather than the therapeutic vector itself. The researchers reported that poor or inconsistent reporting of adverse events (AEs) were a limitation for the meta-analysis. The proportion of patients with any improvement in BCVA and visual sensitivity was 41% (95% CIs of 31% to 51%) and 51% (95% CIs of 31% to 70%), respectively. Systemic immune involvement was associated with a cumulative incidence of 31% (95% CI = 21% to 42%).
Following the analysis of the systematic review, the researchers reported that the pooled proportion of patients with any improvement in vision was 41% (95% CI of 31% to 51%, I2 = 65.62%) and these results were observed over follow-up timelines ranging from 1 month to 7 years, as summarise below:
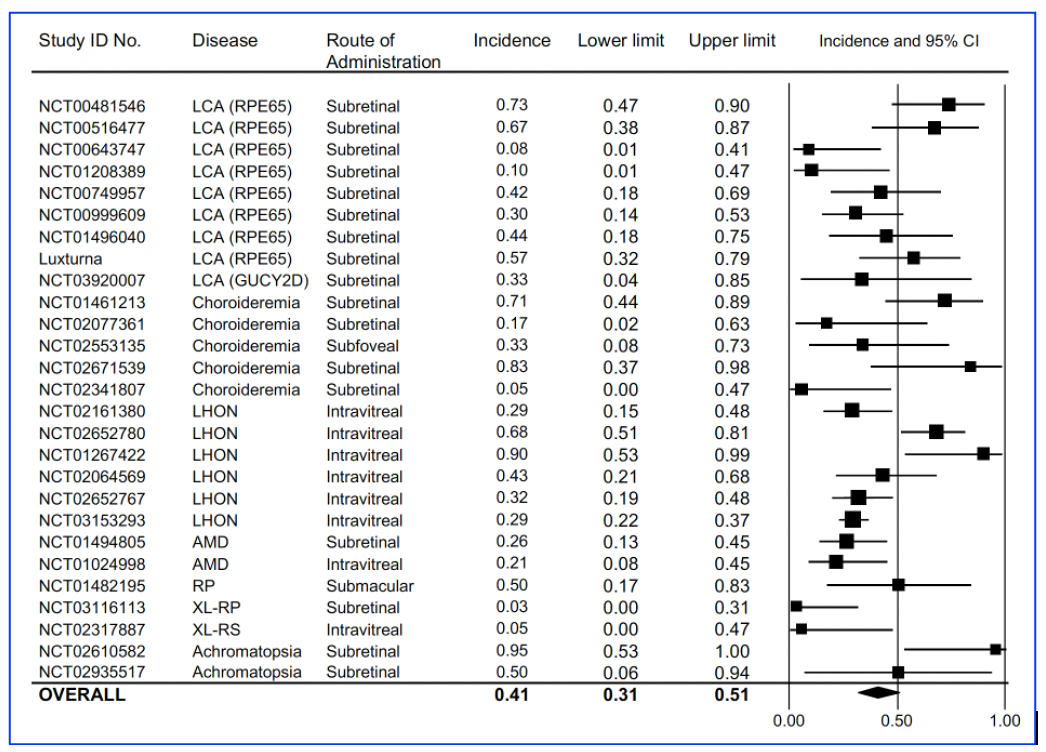
Figure A: Vision improvement using best corrected visual acuity (BCVA) for each of the published clinical trials, published by Sobh et al, entitled, Safety and efficacy of adeno-associated viral gene therapy in patients with retinal degeneration: A systematic review and meta-analysis. Transl Vis Sci Technol. 2023;12(11):24, (https://doi.org/10.1167/tvst.12.11.24).
In addition, the researchers commented on the review stated that the, “report represent 7 distinct diseases, affecting different parts of the retina, and with different modes of inheritance. They used four AAV serotypes, and assessed different promoters, dosages and stages of disease progression. They also used different criteria for evaluations of AEs”. As result, a number of the values of the measures may overestimate the effect, and also underestimate efficacy. The researchers concluded that, “[a]t this time, definitive conclusions are difficult to make, as most gene therapy studies have small sample sizes, lack a control arm, and potentially suffer from methodological bias. Overall, AAV ocular therapy is a promising approach for the treatment of ocular disease. Nevertheless, dosage, site of delivery, volume, and number of injections have to be carefully considered to promote efficacy but avoid AEs”.
