Researchers at the Institute of Molecular and Clinical Ophthalmology Basel (IOB), Switzerland, and the Centre for Medical Genetics, Ghent University, Belgium, have reported results of a study identifying three gene variants – AP5Z1, AP5M1 and AP5B1 – that may cause macular degeneration and impacting sharp central vision. The three genes encode different subunits of the vesicular fifth adaptor protein (AP-5) complex, involved in maintaining cellular homeostasis and the functioning of lysosomal pathways. According to the researchers, the localization and role of the AP-5 complex in retinal tissues have not been studied previously, commenting that, “due to the particular clinical and molecular features observed, we propose designating this phenotype as a distinct clinical subtype of IRD, termed ‘lysosomal macular dystrophy.’”
Inherited retinal diseases (IRDs) may be classified into broad clinical categories including retinitis pigmentosa (RP), cone-rod dystrophy (CRD) or macular dystrophy (MD), and while of ~300 genes have been associated with IRDs, fewer than 20 have been linked to MD and only four to recessive MD. Despite the progress, researchers show that “a considerable proportion of individuals with IRDs remain without a definitive genetic diagnosis, the current molecular diagnostic rate varying between 50% and 80%, mostly as a function of the population studied.” In their current study, a large multi-national collaborative effort involving multiple centres have identified several pathogenic variants in the genes – AP5Z1, AP5M1 and AP5B1 – as an independent cause of a recessive form of retinal disease, “characterized by the progressive loss of macular photoreceptors. This clinical phenotype likely results from defects of the AP-5 complex as a whole at the cellular level, thus broadening the spectrum of previously described adaptinopathies.”
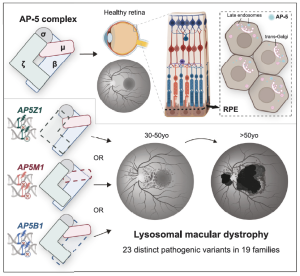
Figure 1. Graphical abstract: Researchers discovered genetic changes in three genes (AP5Z1, AP5M1, and AP5B1) that cause macular degeneration, affecting sharp central vision. The proteins encoded by these genes form a complex that helps the cell’s recycling system clear waste and keep retinal cells healthy. Some affected individuals also experience additional symptoms beyond vision loss. AP-5 is composed of four subunits, zeta, mu, beta, and sigma, encoded by the genes AP5Z1, AP5M1, AP5B1 and AP5S1, respectively.
(This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/), authored by Kaminska et al., entitled, “Bi-allelic variants in three genes encoding distinct subunits of the vesicular AP-5 complex cause hereditary macular dystrophy”, published in The American Journal of Human Genetics, 112, 1–21, April 3, 2025, https://doi.org/10.1016/j.ajhg.2025.02.015).
A total of 22 affected individuals from 19 unrelated families across nine countries, mostly of European descent, were assessed by specialized ophthalmological examinations and using whole-genome sequencing (WGS) performed either as part of the Genomics England 100,000 Genomes project, or the UK NHS Genomic Medicine Service. All patients exhibited a consistent retinal phenotype, with the median age of symptom onset being in their 5th decade. The disease exhibited “flecks at an early stage, incomplete retinal atrophy at an intermediate stage, and complete chorioretinal atrophy extending from the macula toward the periphery at later stages.” All affected individuals carried variants in any of three genes encoding subunits of the AP-5 complex: AP5Z1 (previously associated with spastic paraplegia type 48, SPG48), AP5M1, and AP5B1. The researchers identified 23 unique variants, most of which (74%) were not described previously. AP-5 is composed of four subunits, zeta, mu, beta, and sigma (see figure 1 above), encoded by the genes AP5Z1, AP5M1, AP5B1 and AP5S1, respectively.
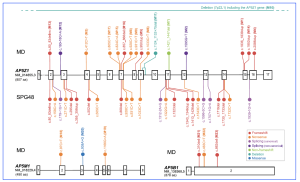
Figure 2. Schematic representation of the genetic variation spectrum of AP5Z1, AP5M1, and AP5B1, with respect to macular dystrophy and spastic paraplegia. The exons of each gene are indicated by numbered boxes, whereas introns are shown by horizontal lines. Their sizes with respect to actual genomic sequence, using canonical transcripts, are roughly proportional, although not to scale. 50 UTRs and 30 UTRs are omitted. The protein size, in number of amino acids (aa), is also indicated. Variants associated with an ocular phenotype (MD, macular dystrophy) and detected in this study (M1 to M23) are shown in various colours above the gene of interest, while variants previously reported in association with spastic paraplegia type 48 (SPG48) are indicated below the gene (only for AP5Z1). These latter DNA changes represent pathogenic or likely pathogenic variants reported in ClinVar as of July 2024, documented in the literature and/or linked to an affected individual. M10, a large deletion affecting the whole AP5Z1 sequence, is indicated by a horizontal line. No clear genotype-phenotype correlation within or across conditions could be detected.
(This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/), authored by Kaminska et al., entitled, “Bi-allelic variants in three genes encoding distinct subunits of the vesicular AP-5 complex cause hereditary macular dystrophy”, published in The American Journal of Human Genetics, 112, 1–21, April 3, 2025, https://doi.org/10.1016/j.ajhg.2025.02.015).
According to the researchers, the fifth adaptor protein (AP-5) complex plays a crucial role in intracellular trafficking and in the sorting and transport of proteins within the late endosome to- Golgi retrieval network. In their study, the authors outlined that “this process is essential for maintaining cellular homeostasis and ensuring the proper functioning of lysosomal pathways. Moreover, the AP-5 complex is thought to be involved in the recovery of lysosomes from endolysosomes, a fundamental process for the maintenance of the balance between different stages of endosomal and lysosomal maturation.” In summary, the research identified bi-allelic variants in genes encoding three of the four subunits of the AP-5 complex (AP5Z1, AP5M1, and AP5B1) “as a previously unreported cause of IRD, with or without extraocular features.” All affected individuals exhibited a specific form of MD, which researchers propose “to classify as a distinct subtype of IRD: lysosomal macular dystrophy.”