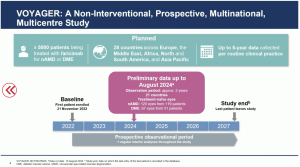
Delegates at the EURETINA Congress in Barcelona (Sept. 20th 2024) have heard new outcome data from a real-world study on faricimab (anti-VEGF, Vabymso), for naïve patients with nAMD and DME, supported by Roche. Dr. Clare Bailey, MD, MRCP, consultant ophthalmologist at Bristol Eye Hospital in Bristol, UK, presented a novel global, prospective, non-interventional study across 26 of 28 countries aimed to collect data and report on clinical and anatomical outcomes in patients initiating faricimab treatment in routine clinical practice (“VOYAGER”). Dr. Bailey reported that faricimab is the first and only intraocular bispecific antibody that independently binds and neutralises angiopoietin-2 and VEGF-A. On 21 July 2022, EMA’s Committee for Medicinal Products for Human Use (CHMP) approved a positive opinion for the medicinal product Vabysmo (faricimab), for the treatment of neovascular age-related macular degeneration (nAMD) and diabetic macular oedema (DME). Previously, results from four phase III studies recruited 3,220 patients, “TENAYA” and “LUCERNE” in nAMD at year one, and “YOSEMITE” and “RHINE” in DME up to two years. The results indicated that patients treated with faricimab, given at intervals of up to four months, achieved non-inferior vision gains and anatomical improvements, compared to aflibercept given every two months. From across all four studies at two years, 60% of patients treated with faricimab were able to extend treatment to every four months, while improving and maintaining vision.
Faricimab is a humanised bispecific immunoglobulin G1 (IgG1) antibody that acts through inhibition of two distinct pathways by neutralisation of both angiopoietin-2 (Ang-2) and vascular endothelial growth factor A (VEGF-A). Ang-2 causes vascular instability by promoting endothelial destabilisation, pericyte loss, and pathological angiogenesis, thus potentiating vascular leakage and inflammation. It also sensitises blood vessels to the activity of VEGF-A resulting in further vascular destabilisation. Ang-2 and VEGF-A synergistically increase vascular permeability and stimulate neovascularisation. By dual inhibition of Ang-2 and VEGF-A, faricimab reduces vascular permeability and inflammation, inhibits pathological angiogenesis and restores vascular stability.
In her presentation in Barcelona, Dr. Bailey told attendees that the real-world study provides ~5,000 patients over a 5-year period for the treatment faricimab of nAMD or DME in routine clinical practice at 330 sites in 28 countries across Asia-Pacific, Europe, North America and South America. Data analysed from November 21st, 2022 to August 2024 included treatment-naïve patients of nAMD (120 eyes from 119 patients) or DME (67 eyes from 51 patients) in 21 countries to date (North America, Australia, Europe and the Middle East).
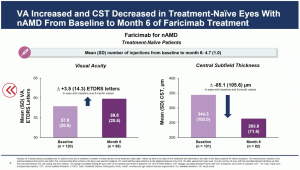
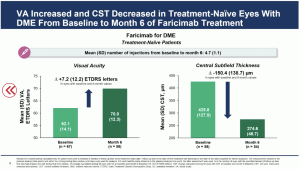
The initial outcomes from the real world data showed a mean VA [SD] increase from baseline to month 6 by +3.5 (14.3) ETDRS letters in eyes with nAMD, and +7.2 (12.2) ETDRS letters in eyes with DME. The mean CST (SD) decreased from baseline to month 6 by -85.1 (105.6) µm in eyes with nAMD, and -150.4 (138.7) µm in eyes with DME.
In conclusion, the real-world VOYAGER investigators commented that this “is ideally positioned to generate widely generalisable data on the effectiveness and safety of faricimab in the real world. The data collected in VOYAGER will be critical to help close gaps in outcomes between clinical practice studies and randomised clinical trials, ensuring optimal long-term outcomes for patients with nAMD and DME globally”.