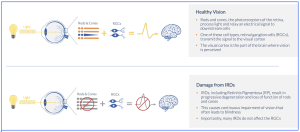
Kiora Pharmaceuticals (NASDAQ: KPRX) have announced the receipt of Orphan Medicinal Product Designation from the European Medicines Agency (EMA) for the treatment of IRDs, including retinitis pigmentosa (RP) and choroideremia. The designation covers “KIO-301” – a small molecule photoswitch, for the treatment of non-syndromic rod-dominant retinal dystrophies. According to the company’s Chief Development Officer, Eric Daniels, M.D., stated that, “this designation provides Kiora and our development and commercialization partner, Théa Open Innovation, with at least ten years of market exclusivity”. In addition to the market exclusivity, Orphan Medicinal Product Designation includes: reduced or waived fees for regulatory activities and EMA scientific advice and protocol assistance to optimize clinical trial design.
KIO-301 is an azobenzene photoswitch compound known to block voltage-gated ion channels, including hyperpolarisation-activated cyclic nucleotide-gated (HCN) and voltage-gated potassium channels during exposure to visible light. The compound is taken up selectively by viable retinal ganglion cells (RGCs) downstream of degenerated photoreceptors, rendering RGCs photoresponsive. KIO-301 delivered intravitreally in patients with retinitis pigmentosa may restore functional vision. Safety data and patient reported outcomes from the first blind human to receive a single low-dose of KIO-301 are reported. KIO-301 (formerly known as B-203) was acquired through Bayon Therapeutics, Inc. (“Bayon”) in 2021, which was previously licensed from the University of California granting the exclusive rights to its pipeline of photoswitch molecules.
Kiora’s clinical trial of KIO-301 (termed “ABACUS-2”), listed at clinicaltrilas.gov (#NCT 05282953), has an official title of, “Phase I/II Dose-escalating Study of the Safety, Tolerability and Efficacy of KIO-301 Administered Intravitreally to Patients With Retinitis Pigmentosa and Choroideremia (ABACUS)”. Kiora also presented data on their website proposing: (Kiora Pharmaceuticals, Inc. NASDAQ: KPRX Q3 2024, Corporate Overview), highlighting a summary schematic on their strategy:
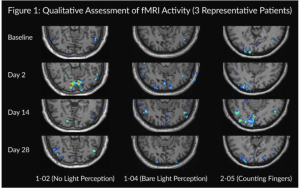
Previously this year, the company additionally reported that KIO-301 significantly increased brain activity, specifically in the visual cortex, relative to baseline, as assessed by functional MRI (fMRI). The results were presented by Professor Robert James Casson, DPhil, Head of the Ophthalmic Research Lab at The University of Adelaide, at the Association for Research in Vision and Ophthalmology (ARVO) annual meeting in Seattle, WA, with additional key findings including:
- a statistically significant increase in visual cortex activity from baseline at all timepoints assessed (1574.0 ± 689.7 voxels at d2, 1061.8 ± 632.1 voxels at d14, 1110.8 ± 478.4 voxels at d28, p<0.05 for all timepoints, n=12).
- a statistically significant increase in visual cortex activity was measured in both cohorts, those with baseline vision of counting fingers or hand motion range and those with baseline vision of bare light perception or no light perception.
- a more pronounced increase in visual cortex activity was found in patients with better baseline vision.
- the increase was time-dependent following initial administration of KIO-301, consistent with improvements in visual acuity, visual field, and functional improvements that mimic performing everyday activities.
Presented online at: https://images.newsfilecorp.com/files/8481/208051_e7fb33683455a9ff_002full.jpg