GenSight Biologics (Euronext: SIGHT, ISIN: FR0013183985, PEA-PME eligible), based in Paris, France, have announced results from a new meta-analyses proposing that those treated with LUMEVOQ (GS010; lenadogene nolparvovec) gene therapy, appear to have experienced a rate of visual recovery “greater than that of idebenone-treated patients and untreated (natural history) patients”. The meta-analyses suggests LUMEVOQ may provide a greater recovery rates than that of idebenone treatment (a synthetic, short-chain analogue of coenzyme Q, which has been reported to be a potential agent to treat LHON), and both greater than that in the natural history of the disease. In addition, the company reported that there was no overlap in confidence intervals when LUMEVOQ was compared to idebenone and to natural history, “indicating a positive difference in visual outcomes”.
Lenadogene nolparvovec is to be used as a novel gene therapy for LHON, an inherited retinal degeneration with has an estimated prevalence of 1 in 40,000 in Europe. GenSight proposes that 1,100 to 1,200 LHON patients may be seeking therapies for this disorder each year. The connection between LHON and mitochondrial DNA (mtDNA) previously arose from studies that reported a homoplasmic nucleotide transition from guanosine to adenosine at position 11778, resulting in an arginine-to-histidine substitution in ubiquinone oxidoreductase (NADH) subunit 4 (ND4) of the mitochondrial complex I. It is now known that the majority of LHON cases are associated with mutations in one of three mitochondrial genes that encode subunits of the same complex I of the mitochondrial respiratory chain. This complex I enzyme, containing 7 subunits encoded by mtDNA, is closely associated with the inner mitochondrial membrane, while a further 35 subunits, encoded by nuclear DNA, are imported into the organelle to facilitate specific steps of the respiratory pathway.
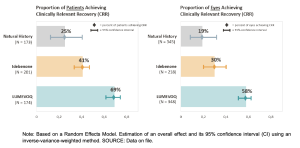
Three meta analyses between natural history, vs. idebenone vs. Lumevoq shows clinically relevant recovery (CRR) measurements presented by the company:
Figure 1 (GenSight, Paris). Visual recovery among ND4 LHON patients [https://www.gensight-biologics.com/]
The results of the meta-analyses were presented at the 2024 annual meeting of the North American Neuro-Ophthalmology Society (NANOS), and the company has proposed the data will be further presented at additional conferences in Europe and the US later this year. Dr. Nancy J. Newman, LeoDelle Jolley Professor of Ophthalmology and Neurology at the Emory University School of Medicine in Atlanta, United-States, presented her findings at the NANOS 2024 conference, commenting that, “[t]his study is very important in order to compare these therapies’ abilities to achieve equivalent end-point metrics. It is clear that the degree of efficacy of the gene therapy was greater than the other currently available options of no treatment and idebenone use as previously administered in studies”. Finally, the company aims to restart an Early Access Program (AAC) in France in Q3 2024 with top-line results at year 4 results of follow-up expected this month.