EyePoint Pharmaceuticals, Inc. (NASDAQ: EYPT), based in Watertown, Massachusetts, have announced positive interim 16-week data for an ongoing Phase 2 “VERONA” clinical trial, evaluating “DURAVYU” (EYP-1901), an investigational sustained delivery therapy of “vorolanib” which acts a tyrosine kinase inhibitor (TKI) for patients with diabetic macular edema (DME). EYP-1901 (ClinicalTrials.gov ID NCT06099184) delivers vorolanib as a solid bioerodible insert, aimed to treat DME (and other VEGF-mediated retinal diseases) as a pan-VEGF receptor inhibitor, inhibiting all VEGF receptors. The proposed treatment will be administered with a standard intravitreal injection in an outpatient setting releasing vorolanib for up to nine months.
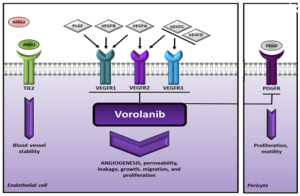
Figure 1([a] and [b]): [a] The company’s summary on their website (https://eyepointpharma.com/pipeline/#sec-1901) proposes an intravitreal injection insert, smaller than the top of a pencil, which will be “immediately bioavailable featuring and initial burst of drug followed by zero order kinetics release for ~9 months”. [b] vorolanib provides a potent and selective pan–VEGF receptor inhibition, including inhibiting PDGF potentially leading to antifibrotic benefit.
The Phase 2 study (VERONA) is a randomized, controlled, single-masked, open label trial for DURAVYU (EYP-1901) in DME patients, previously treated with a standard-of-care anti-VEGF therapy. The study enrolled 27 patients assigned to one of two intravitreal doses of DURAVYU (EYP-1901) (1.3mg or 2.7mg), or aflibercept control. The company outlined a “primary efficacy endpoint of the VERONA trial is time to first supplemental aflibercept injection up to 24 weeks based on established supplement criteria”. Key secondary endpoints include safety, mean change in BCVA, mean change in CST as measured by OCT and change in diabetic retinopathy severity scale (DRSS) over time (identifier: NCT06099184).
The company’s results reported of the 2.7mg dose demonstrated an early, sustained improvement in BCVA and anatomical control versus the aflibercept control arm. In brief there was an improvement in BCVA with a gain of +8.9 letters, compared to +3.2 letters in the aflibercept control group, and an early and sustained anatomical improvement with a 68 micron reduction in CST, versus 30.5 microns in the aflibercept control group. The company additionally reported a favourable safety profile with no DURAVYU (EYP-1901)-related ocular or systemic SAEs to date. Topline data is anticipated to be available in Q1 2025. Following the announcement in a company news release, Jay S. Duker, MD, President and CEO of EyePoint, said, “we are encouraged and excited by these highly positive interim results demonstrating clinically meaningful functional and concomitant structural improvement with a continued favorable safety profile of Duravyu. DME is a prevalent disease with a significant need for more durable treatments. The interim data from the VERONA trial demonstrates that after a single Duravyu 2.7mg treatment, there was a meaningful, early and maintained improvement in BCVA paired with strong anatomical improvement in retinal thickness, demonstrating the potential for Duravyu in DME as a sustained delivery therapy. One of the most notable aspects of these data is that both Duravyu doses showed an immediate benefit over aflibercept control in both BCVA and CST.”