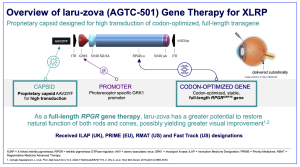
Beacon Therapeutics Holdings Limited, based in London, UK and Cambridge, Massachusetts, have announced the completion of enrolment in its registrational Phase 2/3 VISTA trial evaluating “laru-zova” for the treatment of X-linked retinitis pigmentosa (XLRP). The study has enrolled eligible male patients aged 12 to 50 across sites in North America, the United Kingdom, and Australia. Twelve month topline data from this trial are expected in the second half of 2026. The VISTA trial (#NCT04850118) is a randomized, controlled, masked, multi-center Phase 2/3 study, designed to evaluate the efficacy, safety, and tolerability of laru-zova in male patients with XLRP caused by mutations in the RPGR gene. The primary outcome of the trial is the proportion of participants with a ≥15 letter increase from baseline in LLVA (Low Luminance Visual Acuity) within 12 months. The trial is assessing two dose levels of laru-zova compared to an untreated control group, evaluating the proportion of participants with improvement in LLVA and mean sensitivity as observed by microperimetry, among other measures of visual function.
Figure 1. Presented by Beacon Therapeutics, published available online on https://www.beacontx.com/publications-and-presentations/
“laru-zova”,[AGTC-501] is a gene therapy treatment of XLRP, also referenced to “AGTC-501”, and previously named as rAAV2tYF-GRK1-RPGR. XLRP is one of the most common forms of retinitis pigmentosa with mutations in the RP GTPase regulator gene (RPGR gene), thought to account for approximately 75% of XLRP recorded cases. The gene encodes a ciliary protein that regulates trafficking of proteins to the outer segment of photoreceptors. Males are more severely affected by the X-linked pathology with night blindness generally occurring within the first decade of life, followed by restriction of the visual field and loss of visual acuity leading to legal blindness in most patients by the fourth to fifth decade of life. AGTC-501/laru-zova correctly expresses the full length RPGR protein, thereby addressing the entirety of photoreceptor damage caused by XLRP, including both rod and cone loss.
Previously, Beacon Therapeutics acquired Applied Genetic Technologies Corporation, (previously Nasdaq: AGTC) for €112 million in summer 2023, with AGCT’s clinical-stage adeno-associated virus (AAV)-based gene therapy assets. Beacon now plans to use the data from the VISTA trial, alongside long-term data from ongoing Phase 2 DAWN trial, the Phase 1/2 HORIZON and Phase 2 SKYLINE studies to support regulatory submissions in the United States and Europe. Following the current announcement of the Phase 2/3 enrolment milestone, Dr. Daniel Chung, D.O., M.A., Chief Medical Officer of Beacon Therapeutics, stated that: “the VISTA study has been carefully designed to provide the clinical evidence needed to demonstrate laru-zova’s potential to improve functional vision in patients with XLRP. We are applying our deep understanding of both ocular disease and gene therapy by using a highly efficient AAV capsid and a stabilized gene cassette that expresses the full-length RPGR protein, to support better vision outcomes. We are cautiously optimistic about the potential for laru-zova to treat a condition that has long been considered untreatable.”
Beacon Therapeutics’ team is led by CEO Lance Baldo, MD, previously at Freenome, Adaptive Biotechnologies, Roche Group and Genentech, andl; Daniel Chung, Chief Medical Officer, previously at Sparing Vision, Spark Therapeutics plc., and FM Kirby Center for Molecular Ophthalmology at the University of Pennsylvania Perelman School of Medicine.
Publicly available at https://www.beacontx.com/about-us/