An independent study, funded by the Swedish government, has reported that intravitreal injections of anti-VEGF treatments were associated with a 2.9% risk for stroke among patients with neovascular age-related macular degeneration (nAMD). The risk was higher with aflibercept and bevacizumab than ranibizumab and the risk declined 60 days after the last injection. Several previous trials had reported the safety of intravitreal injections revealing detectable levels in the systemic circulation from all three anti-VEGF treatments (ranibizumab, aflibercept and bevacizumab) however, results were inconclusive. The current Swedish work used an observational study by linking the data from the Swedish Macula Register (SMR) with the Swedish Stroke Resister (“Riksstroke”). Both of the registries were large national population-based quality registries with excellent coverage, 85%–90% (Macula, 2020; Riksstroke, 2020) and this provided a valuable methodology to interrogate the data sets.
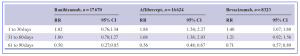
According to the researchers, Riksstroke had 409,546 stroke patients, while SMR had 33,585 nAMD patients who have received intravitreal anti-VEGF injections over a 12-year period. Of the total number of injections, 26,263 were unilateral and 6,555 were bilateral treatment. The cross-linking of the two databases showed that 1,693 patients had a stroke, and among those patients, 1,330 received ranibizumab, 503 received aflibercept and 314 received bevacizumab. Of all stroke incidents, 936 occurred within 90 days of the last treatment, 527 within 30 days, 295 within 31–60 days and 114 within 61–90 days.
Table 1*. Incidence of stroke within 30, 31–60 and 61–90 days of last Anti-VEGF treatment in SMR with Ranibizumab, Aflibercept and Bevacizumab.(RR, risk ratio; CI, confidence interval).
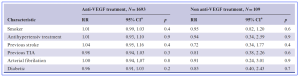
Table 2*: RR for a stroke within 90 days for stroke patients on anti-VEGF treatment and Non anti-VEGF treatment. (RR, risk ratio; CI, confidence interval; p, probability).
*The research work is licensed under a Creative Commons Attribution-Non Commercial-No Derivatives 4.0 International License, cited by Falemban, A.H., et al, (2025), entitled: “Intravitreal anti-vascular endothelial growth factor injections and risks of stroke in patients with neovascular age-related macular degeneration—A registry-based cohort study. Acta Ophthalmologica, available from: https://doi.org/10.1111/aos.17534.
The study results showed that, compared with non-use, intravitreal anti-VEGF agent was associated with an increased risk of stroke within 90 days of anti-VEGF treatment in 2.9% of the nAMD-patients [Risk Ratio (RR) 1.27, 95% confidence interval (CI) 1.22; 1.33], compared to non-users. The RR within 30, 31–60 and 61–90 days were 1.36 (1.15; 1.66), 1.40 (1.09; 1.79) and 0.58 (0.52; 0.65), respectively. The researchers commented that, “even though the risk is small, intravitreal injections with anti-VEGF agents for the treatment of nAMD are associated with an increased risk of stroke/TIA. The risk seems to be higher within 60 days of last injection. An assessment of high-risk populations and risk-benefit weighting is necessary before intravitreal anti-VEGF injections are considered.” In addition, the authors commented that, “these variations in stroke risk between the agents in our study need to be interpreted with caution as our data were not able to determine whether they were caused by confounding by indication or by an actual risk difference. The majority of our study cases (78.5%) were treated with ranibizumab, which could be partially explained by the fact that ranibizumab was the only anti-VEGF agent used in Sweden between 2006 and 2011.”