A large real-world prospective study on the long-term effectiveness and safety of faricimab and “Port Delivery System with ranibizumab” (PDS), for nAMD or DME, is to run to the end of Dec. 31st, 2027. Enrollment began in November 2022, with more countries expected to initiate recruitment through to 2024. The purpose of the study is “to understand drivers of clinical practice treatment outcomes by gaining novel insight into the intersection of treatment regimens, decisions, anatomic outcomes, and vision”. The primary objective of the study is to evaluate the clinical practice effectiveness of faricimab and PDS, and the primary end point will assess VA (visual acuity) change (approximate ETDRS letter score) from baseline at 12 months (nearest visit to 12 months). A key secondary end point will assess VA change over designated timepoints (nearest visits to 3, 6, 24 months, and annually thereafter). The “final analysis will be conducted after the last patient has exited the study” and “exploratory interim analyses of selected end points are planned to be performed annually or semiannually (depending on available data)”.
Faricimab (brand name Vabysmo), is a monoclonal antibody used for the treatment of nAMD and DME as the first bispecific monoclonal antibody targeting both vascular endothelial growth factor (VEGF) and angiopoietin 2 (Ang-2). It was approved by the FDA in January 2022, and subsequently in the EMA in September 2022. PDS is a drug delivery system designed for the continuous delivery of ranibizumab into the vitreous for 6 months and beyond. The PDS includes an ocular implant, a customized formulation of ranibizumab, and four dedicated ancillary devices for initial fill, surgical implantation, refill-exchange, and explantation, if clinically indicated. The PDS system was approved by the FDA in 2021.
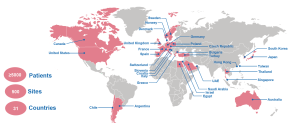
In the real-world study (named “VOYAGER”), there are 500 sites across 31 countries with an excess of >5,000 patients to be recruited:
Fig. 1. Countries with sites participating in the VOYAGER study. UAE = United Arab Emirates. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/ licenses/by-nc-nd/4.0/); Guymer et al., Rationale and Design of VOYAGER: Long-term Outcomes of Faricimab and Port Delivery System with Ranibizumab for Neovascular Age-Related Macular Degeneration and Diabetic Macular Edema in Clinical Practice; Ophthalmology Science Volume 4, Number 3, June 2024, https://doi.org/10.1016/j.xops.2023.100442. .
While anti-VEGF treatments and regimens have been a paradigm shift on the management of age-related retinal degenerations, outcomes outside of the RCT process can differ and/or impact on long-term effects that may not maintain the original efficacy measures. There can be multiple factors on these outcomes, ranging from inadequate treatment frequency to design, treatment philosophies, access, socioeconomic factors and others. The current researchers have commented that “these factors need to be assessed to implement appropriate strategies that can help close the gap between clinical practice and clinical trial outcomes”.
The real-world study “will be per usual care, with no mandated scheduled visits/imaging protocol requirements”. While the primary end point measures VA change from baseline at 12 months per study cohort (faricimab in nAMD and in DME, PDS in nAMD), there are 17 secondary end points, including, “VA change over time and per treatment regimens (fixed, treat-and-extend, pro re nata, and other) and number. Exploratory end points includes, VA change in relation to presence/location of anatomic features that impact vision (fluid, central subfield thickness, fibrosis, atrophy, subretinal hyperreflective material, diabetic retinopathy severity, and disorganization of retinal inner layers) and per treatment regimen/philosophies. The impact of regional and practice differences on outcomes will be assessed as will safety”.
In conclusion, VOYAGER is “an innovative study that will evaluate long-term vision outcomes and safety of faricimab and PDS for the treatment of nAMD and DME in routine clinical practice at global and regional levels”. The researchers have commented that, “VOYAGER is ideally positioned to identify novel and potentially addressable factors affecting outcomes. The novel investigator interface will provide clinicians with a holistic picture of each patient’s disease evolution over time to help inform treatment decisions. The data collected in VOYAGER will be critical to helping close the gap in outcomes between clinical practice studies and clinical trials and ensuring optimal long-term outcomes for patients with nAMD and DME globally”.