A Phase 3 randomised clinical trial has recruited of >400 patients in a seven-month period, driven by clinical and patient interest, according to the trial’s sponsor EyePoint Pharmaceuticals, based in Watertown, Massachusetts, United States. The Phase 3 trials, named “LUGANO” and “LUCIA”, are both global, randomized, double-masked, aflibercept controlled, non-inferiority trials, assessing the efficacy and safety of the vorolanib intravitreal insert. Vorolanib (also named “EYP-1901, 2,686 µg) is a tyrosine kinase inhibitor (TKI) as a solid bio-erodible insert aimed to intracellularly inhibit pan-VEGF and VEGFR, designed to last for 6-months, thereby minimising anti-VEGF injections over the course of the treatment. Following the sponsor’s announcement of the recruitment rate, Jay S. Duker, M.D., stated that “we are committed to bringing the first sustained-release tyrosine kinase inhibitor (TKI) to market for patients and physicians in need of a new treatment option for wet AMD. With both pivotal trials continuing to exceed our timelines, we now expect topline data for the LUGANO trial in mid-2026 with LUCIA to follow in the second half of 2026.”
LUGANO dosed the first patient in October 2024, while LUCIA dosed the first patient in December 2024 (the LUGANO identifier at clinicaltrials.gov is NCT06668064 and LUCIA identifier is NCT06683742). The primary endpoint of the Phase 3 pivotal trials is the average change in BCVA at weeks 52 and 56 versus baseline and secondary endpoints including safety, reduction in treatment burden, and the “percentage of eyes free of supplemental aflibercept injections and anatomical results as measured by optical coherence tomography (OCT)”, as reported by the sponsor. Vorolanib was developed as a potential sustained-delivery maintenance treatment for patients from chronic VEGF-mediated retinal disease. Previous Phase 1 and 2 results reported clinically meaningful efficacy with stable visual acuity and CST presenting an ”impressive treatment burden reduction of approximately 88% six months after treatment” and with “>80% of patients supplement-free or receiving only one supplemental anti-VEGF injection.”
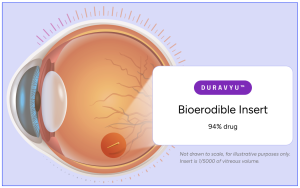
Figure 1. Vorolanib is a selective and patent protected tyrosine kinase inhibitor that provides a new mechanism of action and treatment paradigm for retinal diseases beyond existing anti-VEGF large molecule ligand blocking therapies. The technology, termed “DURAVYU™” is an investigational product utilizes a bio-erodible insert technology licensed to EyePoint Pharmaceuticals, exclusively by Equinox Sciences, a Betta Pharmaceuticals affiliate for the localized treatment of all ophthalmic diseases outside of China, Macao, Hong Kong and Taiwan(https://eyepointpharma.com/science-and-technology/).
According to the sponsor, both Phase 3 studies enroll >400 patients each, for LUGANO and LUCIA, randomly assigned into two groups and receive either vorolanib 2.7 mg, or an on-label aflibercept control. Patients in the treatment arm will receive a 2.7-mg intravitreal injection every 6 months beginning at month 2 of the trial. Commenting on the announcement, Brittney Statler, M.D., Principal Investigator in the LUGANO clinical trial and Medical Retina Specialist at Panorama Eyecare, Fort Collins, Colorado, stated that “the pace of enrollment in the LUGANO trial highlights EyePoint’s engagement with the retinal community and underscores the enthusiasm for this patient-centric pivotal program for DURAVYU. One of the many compelling elements of this program is the fact that all patients receive active treatment with either aflibercept or DURAVYU, enabling us to effectively evaluate this potential next generation treatment for wet AMD in a real-world clinical practice setting. We are honoured to be part of this innovative clinical research for a treatment that has the potential to change the current treatment paradigm and revolutionize clinical outcomes for patients suffering from serious retinal diseases.” The completion of enrollment for LUGANO’s topline data is anticipated in mid-2026.