Clinical researchers at the Sorbonne Université, INSERM, CNRS, Institut de la Vision and the Centre Hospitalier National d’Ophtalmologie des Quinze-Vingts, Paris, have reported PCARE-associated retinopathy as a severe generalized photoreceptor dystrophy, with macular atrophy. While the visual field loss occurs early with the study population, useful central vision may be retained into late adulthood due to of foveal sparing (FS). The study’s results confirm “the hypothesis that the disease mechanism stems from a complete loss-of-function of the PCARE protein in photoreceptor cilia. Furthermore, we identified 11 novel PCARE variants, expanding the array of about 80 published variants already associated with a clinical phenotype.” Despite that there is no treatment currently exists for PCARE-associated retinopathy, researchers hope that an ideal candidate for gene therapy development may be viable. Given that gene augmentation may be the most suitable strategy for recessive conditions, PCARE-associated retinopathy may have an optimal therapeutic window possible to before the fifth decade of life.
Researchers have outlined that the photoreceptor cilium actin regulator (PCARE) gene was first identified as a cause of non-syndromic autosomal recessive retinitis pigmentosa (RP54) in 2010. The disorder is characterized by typical signs of RP, including poor night vision and peripheral field loss, retinal bone spicule-type pigment deposits, pale optic discs, and markedly reduced or extinguished responses on electroretinography. The gene encodes a 1,289-amino acid protein predominantly expressed in the primary cilium of retinal photoreceptors, regulating outer segment disk formation and renewal. In their current research, a retrospective cohort study evaluated 28 patients (56 eyes) with main outcome measures of best-corrected visual acuity (BCVA) and degree of vision impairment, kinetic visual field (KVF), area of macular atrophy (MA) with definitely decreased autofluorescence (DDAF) on short-wavelength autofluorescence, total macular volume (TMV) and foveal sparing (FS) on optical coherence tomography.
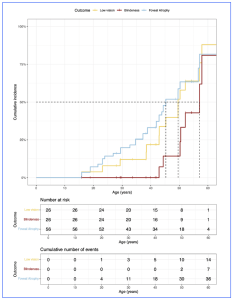
Figure 1. Time-to-event curves for development of foveal atrophy, low vision, and blindness in PCARE-associated retinopathy. The median age for the loss of foveal sparing is 45 years, while the median ages for the development of low vision (best corrected visual acuity in the better-seeing eye worse than 0.5 [logMAR] or 20/70 [Snellen]) and blindness (best-corrected visual acuity in the better-seeing eye worse than 1.3 [logMAR] or 20/400 [Snellen]) are 50 and 57 years, respectively. [This figure is provided from an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license: http://creativecommons.org/licenses/by/4.0/, authored by Bianco, L et al., entitled, “PCARE-Associated Retinopathy – Genetics, Clinical Characteristics, and Natural History”, published in Invest Ophthalmol Vis Sci. 2025;66(4):61. https://doi.org/10.1167/iovs.66.4.61].
The results of the natural history of the PCARE-associated retinopathy showed a phenotype consistent with a severe generalized photoreceptor dystrophy, with macular atrophy and with DDAF observed in 85% of the eyes the study population. Loss of foveal sparing (FS) (occurring at a median age of 45 years) was associated with a mean BCVA (logMAR) worsening by 1.1 (95% confidence interval [CI] = 0.6 to 1.5, P < 0.001) and low vision and blindness in the better-seeing eye occurred at median ages of 50 and 57 years, respectively. The comprehensive natural history is key to support subsequent experimental treatments in the coming years. The recent study results, collected longitudinal data over an average follow-up of almost 7 years, will likely capable for exploring potential functional and structural outcome measures for upcoming clinical trials. In concluding their work, the clinicians commented that, “none of the patients experienced blindness in both eyes before the age of 40 years. These findings suggest that the optimal therapeutic window for future gene augmentation approaches might be before the fifth decade of life.”