A new real-world study, led by Massachusetts General Hospital, Harvard Medical School, Boston, US, have reported a major adverse event on hydroxychloroquine retinopathy, with an ocular toxic effect that can develop over long-term use. Hydroxychloroquine is an efficacious treatment for rheumatic diseases, and a mainstay for patients with systemic lupus erythematosus (SLE) however, over long-term use of the treatment can lead to retinal toxic effects, thinning of the outer retina and eventual damage of the retina pigment epithelium (RPE). The results of a longitudinal cohort of 4,677 patients in northern California has suggested that dosing decisions need to be evaluated in the context of an association with increasing age, female sex, chronic kidney disease (CKD) stage and tamoxifen use, indicating a higher risk of hydroxychloroquine retinopathy while participants younger than 45 years (at hydroxychloroquine initiation) and male sex appear to have a lower risk.
Hydroxychloroquine is a medication used to treat rheumatoid arthritis, lupus and porphyria cutanea tarda (including for the prevention and treatment of malaria). It was originally approved for medical use in the United States in 1955 and it is on the World Health Organization’s List of Essential Medicines as the 116th most commonly prescribed medication. The treatment is a relatively low-cost prescription, often sold under the brand name Plaquenil, among others, frequently sold as a sulfate salt (hydroxychloroquine sulfate). In their study of long-term hydroxychloroquine users, the summary demographic data showed82.9% were female, and 17.1% were male, and the mean [SD] age at hydroxychloroquine initiation was 52.4 (14.1) years. Although many patients (2858 [61.1%]) initiated a hydroxychloroquine dose over 5mg/kg/d, the mean (SD) initial weight-based dose was 4.4 (1.5) mg/kg/d. By 5 years of use, only 1608 (34.4%) were using a hydroxychloroquine dose over 5mg/kg/d, with a mean (SD) dose of 3.2 (1.9) mg/kg/d. Following their anaylsis, 125 patients developed hydroxychloroquine retinopathy within 15 years (102 parafoveal, 23 pericentral). Older age at time of hydroxychloroquine initiation was associated with retinopathy risk, with adjusted hazard ratios (HRs) of 2.48 (95%CI, 1.28-4.78) for those aged 45 to 54 years, 3.82 (95%CI, 2.05-7.14) for those aged 55 to 64 years, and 5.68 (95%CI, 2.99-10.79) for those aged 65 years or older compared with those younger than 45 years. The risk of retinopathy was higher among females than males (HR, 3.83 [95%CI, 1.86-7.89]), among patients with CKD stage 3 or greater (HR, 1.95 [95%CI, 1.25-3.04]), and among individuals with tamoxifen use (HR, 3.43 [95%CI, 1.08-10.89]). The likelihood of pericentral retinopathy was higher among Asian patients (HR, 15.02 [95%CI, 4.82-46.87]) and Black patients (HR, 5.51 [95%CI, 1.22-24.97]) compared with non-Hispanic White patients.
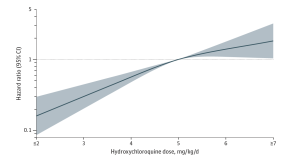
Figure 1. Weight-Based Hydroxychloroquine Dose and the Risk of Hydroxychloroquine Retinopathy After 15 Years of Use. The curve was constructed using a multivariable Cox proportional hazards regression model, adjusting for age, sex, race and ethnicity, indication for hydroxychloroquine use, and chronic kidney disease. Hydroxychloroquine dose was assessed at 5 years of hydroxychloroquine use. Hydroxychloroquine retinopathy was assessed through 15 years of follow-up. The smoothed curve was fitted with restricted cubic splines with knots at 4, 5, and 6 mg/kg/d. The reference weight-based dose was 5 mg/kg/d. The horizontal dashed line indicates a hazard ratio of 1.0. The shaded region indicates the bounds of 95%CIs for the restricted cubic spline curve. [Open Access article, under a Creative Commons Attribution 4.0 International License (visit http://creativecommons.org/licenses/by/4.0/), Jorge et al, JAMA Network Open. 2024;7(5):e2410677].
In follow-up to their analysis, the researchers commented that “the retina is known to thin with age, and these age-related changes may render older individuals more vulnerable to the toxic effects of hydroxychloroquine. CKD stage 3 or greater was also found to be a risk factor for hydroxychloroquine retinopathy. Given the important role of the kidneys in clearance of hydroxychloroquine, renal insufficiency likely leads to a patient being effectively exposed to a higher systemic dose of the medication”. In conclusion, these findings are “relevant to long-term users of this important and commonly prescribed medication for patients with SLE and other rheumatic and dermatologic conditions. Female sex, older age, CKD stage 3 or greater, and tamoxifen use were each associated with an increased risk of hydroxychloroquine retinopathy, whereas male sex, younger age, and normal kidney function were associated with a lower risk of hydroxychloroquine retinopathy. These factors should influence hydroxychloroquine dosing and monitoring for this complication”.