An international clinical research consortium has reported recommendations on endpoints and design of clinical trials in USH2A-related retinal degenerations. The study, supported by the US Foundation Fighting Blindness (FFB), was evaluated on static perimetry, microperimetry, visual acuity, full-field stimulus testing (FST) and optical coherence tomography. Their results and recommendations concluded that the rate of change of mean sensitivity on static perimetry for these USH2A patients have a sensitive perimetric continuous measure, and percentages of within-eye changes were largest with FST testing and microperimetry. Given the outcomes, the researchers’ results will likely affect clinical trial design in the future for USH2A-related retinal degenerations, and potentially other IRDs. These natural history data will be highly relevant to inform eligibility criteria and endpoints for pending clinical trials.
Usher syndrome (USH) is a rare disease, historically categorised into three groups (USH1, USH2, and USH3), dependent on the severity of hearing loss, onset of retinitis pigmentosa (RP) and presence/absence of vestibular dysfunction. (A fourth and atypical subgroup (USH4) are further identified with variants in arylsulfatase G (ARSG) centrosomal proteins (CEP78 and CEP250), and abhydrolase domain-containing protein 12). USH may arise from mutations in at least 9 genes including: MY07A, USH1C, CDH23, PCHD15, USH1G, USH2A, GPR98, WHRN and CLRN. In respect of this initial project for this international consortium, a natural history study of patients with biallelic disease–causing variants in the USH2A gene are one of the most common causes of IRDs. The resulting rod–cone degeneration may be accompanied by congenital hearing loss (Usher syndrome type 2 [USH2]) and vision loss typically begins with nyctalopia followed by midperipheral visual field loss and slowly progresses toward both the center and periphery, often with a prolonged period of preservation of central vision.
In terms of evaluating clinical trial endpoints for USH2A patients (or non-syndromic autosomal recessive retinitis pigmentosa [ARRP]), one of the significant challenges is to how best to evaluate relevant outcomes supportive for real-world outcomes for USH2A patients. In the current study, the consortium recruited USH2A patients or ARRP with biallelic disease-causing variants, visual acuity ≥ 20/80, and visual field ≥ 10° diameter in a 4-year natural history study. Rates of change estimated from mixed-effects linear models and percentages of eyes with changes exceeding the coefficient of repeatability (CoR) or thresholds conforming with FDA guidelines. According to the researchers. “rates of change were generally more sensitive to change than proportions of eyes exceeding a threshold such as the CoR. Baseline ellipsoid zone area ≥ 3 mm2 was necessary to detect change. Mean sensitivity and volumetric hill of vision measures on static perimetry had similar properties and were the most sensitive to changes of the continuous measures. The highest 4-year proportions of eyes exceeding the CoR were from FST testing (47%) and microperimetry (32%). Specification of loci as functional transition points (FTPs) resulted in 45% (static perimetry) and 46% (microperimetry) at 4 years, meeting FDA guidelines for progression”.
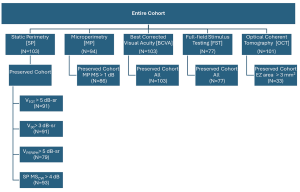
Figure 1. Participants (up to n=105) included in analyses for each measure. Shown are the number of participants in the entire cohort and the preserved cohort for each measure and testing modality. VTOT is the total volume beneath the surface of the hill of vision, V30 is the volume below a central region with radius of 30°, and VPERIPH is VTOT – V30.
The consortium’s purpose was to provide the results of a comprehensive evaluation of the measures for suitability as outcome measures in clinical trials for USH2A-related retinal degeneration. The full recommendations of the research were published in Translational Vision Science & Technology (2024;13(10):15), and also concluded that the ”evaluation of treatments for USH2A-related retinal degeneration (and the vast majority of other IRDs) faces the challenges of a relatively limited population of patients, slow rates of progression, and high variability in the rates of progression across patients, even within a group with disease-causing variants in the same gene. Use in clinical trials of outcome measures that are sensitive to change and clinically important is required to identify beneficial interventions”.