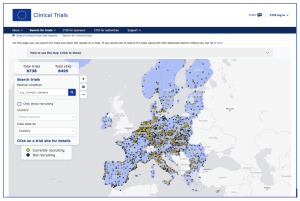
EMA (European Medicines Authority) officials have launched a new clinical trial map aimed to provide access to the public for “patients and healthcare professionals with easy access to comprehensive, real-time information about clinical trials conducted in their area, increasing access to clinical research in the European Union (EU).” EMA’s new map is an action of the “Accelerating Clinical Trials in the European Union (ACT EU)” initiative workplan for 2025-2026 (https://accelerating-clinical-trials.europa.eu/index_en ). The purpose of the initiative is to provide a simple, patient-friendly dashboard for the Clinical Trials Information System (CTIS) to help stakeholders, particularly patients, locate clinical trials of interest in Europe. At present, the map show 8,738 clinical trials located across the EU.
Figure 1.: The “Trial Map” may be located at: https://euclinicaltrials.eu/search-for-clinical-trials/trial-map/?lang=en and a video recording may be found at the EMA website to introduce the integration of the map of clinical trials in the EU, with the CTIS public portal:https://www.ema.europa.eu/en/events/finding-clinical-trials-act-eu-trial-map#:~:text=Live%20broadcast,Event%20summary,with%20the%20CTIS%20public%20portal
CTIS is the single-entry point for sponsors and regulators for the submission and assessment of applications for clinical trials in the EU. The system includes a public searchable database for healthcare professionals, patients and the general public to deliver a high level of transparency foreseen by the EU Regulation (EU) No 536/2014 (CTR). The CTR applies to interventional clinical trials with an investigational medicinal product (IMP), responsible for the assessment, authorisation and supervision of clinical trials running in their territory. The European Commission oversees the implementation of the Clinical Trials Regulation, while EMA is responsible for maintaining the Clinical Trials Information System (CTIS). The Trial Map is integrated in the CTIS public portal and only includes the clinical trial data that is published in accordance with the Clinical Trials Regulation.
The Trial Map, developed through the ACT EU office, will support smarter clinical trials through regulatory, technological and process innovation. ACT EU, launched in January 2022 by the European Commission, the EMA and Heads of Medicines Agencies (HMA), commented that, “our vision is to transform the EU into a region that supports clinical trial development and enables collaboration and innovation at all stages of the clinical research lifecycle. Seamless coordination among stakeholders, regulators and ethics committees will lead to more cross-border collaboration.” The EMA believes the initiative will provide an improved and more impactful clinical trials system, benefitting patients and healthcare in Europe in the process.
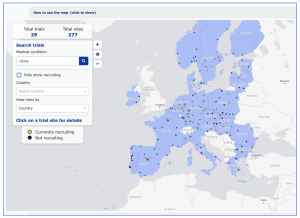
A brief search of the Trial Map for studies on “retina” shows a 29 clinical trials with 277 clinical sites across the EU:
In addition, according to the ACT EU office, the work provides a multi-annual programme aiming to create a “favourable environment for research and development in life sciences, through harmonisation, innovation and collaboration with stakeholders”. The work builds on momentum of the Clinical Trials Regulation (CTR) and the launch of the Clinical Trials Information System (CTIS) on 31 January 2022. The initiative will deliver on the clinical trial innovation recommendations of the European medicines agencies network strategy and the European Commission’s Pharmaceutical strategy for Europe.