Clinical researchers at the Department of Ophthalmology, Bascom Palmer Eye Institute, in the University of Miami, Florida, have presented an update on clinical trial endpoints and an overview of stage 2 and 3 IRD trials, through to the second quarter of 2024. The researchers highlighted that there has been a surge of interest in recent years in IRD trials due to considerable technological advances and significant unmet need, not least of all an estimated cost for healthcare and individual productivity losses between $13 to $32 billion annually in the US. Given that there is a considerable variety on genotypic and phenotypic heterogeneity, rates of progression of IRDs from mild to severe, and new research techniques on measuring outcomes, the researchers outline a timely update on the current space.
Their paper showed that IRDs affect an estimated 184,000 to 438,000 people in the United States, and an estimated 5.5 million people worldwide. The top five most common IRD genes identified were ABCA4 (12.9%), USH2A (6.8%), RPGR (2.7%), EYS (2.1%), and RHO (1.9%).
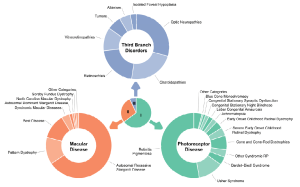
Figure 1. Graphical depiction of the distribution of 1000 consecutive probands among the larger diagnostic categories. Reprinted from publisher—Stone et al. [Clinically Focused Molecular Investigation of 1000 Consecutive Families with Inherited Retinal Disease. Ophthalmology 2017, 124, 1314–1331]. The center chart indicates the proportion of probands assigned to each of the three main branches of the classification system. The outer charts show the fraction of probands assigned to the larger diagnostics categories within each branch. (The article referenced below is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https:// creativecommons.org/licenses/by/ 4.0/), Igoe, J.M et al., Update on Clinical Trial Endpoints in Gene Therapy Trials for Inherited Retinal Diseases. J. Clin. Med. 2024, 13, 5512. https:// doi.org/10.3390/jcm13185512)
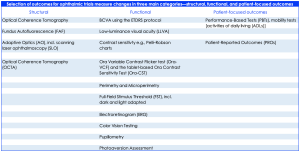
Outcomes for ophthalmic trial measures change in three main categories—structural, functional, and patient-focused outcomes. In respect of IRD endpoints, there are a wide range of specific primary and secondary outcomes however, primary endpoints in the current update on phase 2, 2/3, and 3 gene therapy trials include a mean change from baseline in best corrected visual acuity (BCVA), microperimetry, and/or ambulatory navigation/mobility mazes. Secondary outcomes have included low-luminance visual acuity (LLVA), visual field, full-field light sensitivity threshold (FST), visual perception by quality of life (QoL) questionnaires, electroretinogram (ERG), microperimetry, fundus photography, nystagmus testing, central retinal thickness as measured by optical coherence tomography (OCT), pupillary light reflex response, and photophobia testing.
Table 1. Summary of outcome measurements – structural, functional and patient-focused outcomes
The researchers commented that, “genetic engineering and gene therapy techniques continue to advance with growing investment from industry”, and in addition, vocal patient organizations are keenly aware of outcome measurements in a moving landscape. IRDs are broad, from mild to severe, and a measurement on improving a change of > 0.3 LogMar needs to contextualize the measure in respect of a specific pathology.
An update, as of July 2024, showed that completed and ongoing phase 2, 2/3, and 3 trials reported eight indications: biallelic RPE65-associated retinal dystrophy, CEP290-mediated LCA10, USH2A-mediated RP, RPGR-mediated, XLRP, RP (unspecified), Stargardt, MT-ND4-mediated LHON, and choroideremia, across Europe, Asia and USA. The comprehensive update detailed each of the trials providing a valuable resource for current clinicians. The study showed that the clinical trial environment is quite dynamic. Functional endpoints are prevalent in the short term but they may likely move away from the MLMT (multi-luminance mobilty test) assay to more sensitive mobility assessments like the Luminance Dependent Navigation Assessment (LDNA), developed by Ocugen and supported in collaboration with the FDA. Structural endpoints with FAF acting as an outcome can easily measure atrophic changes, and EZ (ellipsoid zone) measurements by OCT will standardize the quantification for trials in the coming years. Finally, in terms of patient-focused outcomes, well-designed and validated questionnaires will continue to provide key insights into the “real-world” dimension, aimed to support a range of objective and subjective measures.