Researchers at the Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, Milan, Italy and the Department of Ophthalmology, University of Oslo, Norway, have published a systematic review on retinitis pigmentosa (RP) and therapeutic approaches. The review was aimed to evaluate the efficacy and safety of emerging treatment modalities for RP, including mesenchymal-cell-based approaches, gene therapy, pharmacological interventions, nutritional supplements, neuroprotective agents and supplementary interventions. The researchers commented that a “comprehensive data synthesis was unavailable due to the diversity of the available data and differences in research designs”. As a result, the outcomes were not measured through standard meta-analyses. The review evaluated RP, autosomal dominant RP, “advanced” RP, X-linked retinitis pigmentosa and biallelic RPE mediated inherited retinal disease,
The search collected 13 studies comprising a total sample study to 917 eyes covering mesenchymal stem cells (MSC), AAV gene therapy, oral supplementation DHA (docosahexaenoic acid), oral VPA (valproic acid), oral QLT091001(9-cis-retinyl acetate, a late-stage synthetic retinoid replacement treatment) and a retina implant device (Alpha IMS).
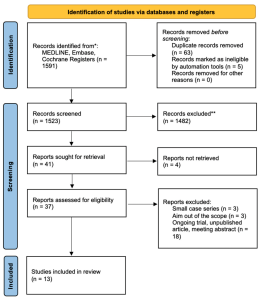
Figure 1. Flowchart of the literature search and selection according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). * Consider, if feasible to do so, reporting the number of records identified from each database or register searched (rather than the total number across all databases/registers). ** If automation tools were used, indicate how many records were excluded by a human and how many were excluded by automation tools. [Confalonieri, et al. Retinitis Pigmentosa and Therapeutic Approaches: A Systematic Review. J. Clin. Med. 2024, 13, 4680; the article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https:// creativecommons.org/licenses/by/ 4.0].
The review reported different evaluations on MSC treatments, including statistically significant results on BCVA and visual field tests and with short-term therapeutic effects. Gene therapy reported certain gains in visual function (with XLRP) and in RPE65-mediated IRD and other results showed, “consistent but insignificant improvements in ambulatory navigation, light sensitivity, and visual field”. DHA applied in XLRP patients reported that, “no significant group differences were found for visual acuity, shape discrimination, or fundus appearance, DHA supplementation showed substantial reductions in the yearly progression rates for dark-adapted thresholds and visual field sensitivity in various regions (p = 0.06 to <0.0001)”. Oral valproic acid (VPA), used at doses ranging from 500 mg to 1000 mg daily showed that the “study did not meet its primary endpoint, as there was no significant change in visual field area between the VPA and placebo groups at 12 months”. Oral QLT091001 was used for patients with RP resulting from mutations in the RPE65 or LRAT genes and these show, “within two months of treatment, 44% of participants experienced a 20% increase in the functional retinal area, with 22% showing a 40% increase”. Finally, with the retinal implant Alpha IMS device, late stage RP patients reported 72% “enhancements in their daily activities and mobility, indicating a substantial improvement in their quality of life”. The research used a “GRADE” system, designed as a tool to assess the integrity of evidence, showing that seven of the thirteen studies reported being of “high quality”, 5 studies were “moderate” and 1 study was considered to be as “low” quality.
In conclusion, the researchers commented that there was, “substantial gaps in evidence and heterogeneity in study methodologies”, and this underscores the need for “further research to elucidate optimal treatment strategies, refine patient selection criteria, and enhance long-term outcomes”. In addition, there are other systematic reviews in the literature with meta-analyses allowing comparisons between, for example, different anti—VEFG treatments in other diseases (including AMD), or particular IRDs on a given disorder (with a specific mutation), using a particular type of treatment and these may facilitate analyses.