Researchers at the Department of Ophthalmology, Vagelos College of Physicians and Surgeons, Columbia University, New York, have published a study outlining how fundus autofluorescence (FAF) underscores a critical role of imaging in both diagnosis and longitudinal care of patients with IRDs. The non-invasive techniques provides valuable insights, including benefits in detecting subclinical or early-stage IRDs and monitoring disease progression over time. The researchers showed areas of decreased autofluorescence correlate with disease progression for certain dystrophies and have been proposed as a biomarker for future clinical trials. In addition, FAF may also help differentiate Stargardt disease from other macular dystrophies and provide benefit in tracking progression of choroideremia and help identify disease carrier status. FAF also improves the characterization of mitochondrial retinopathies such as maternally inherited diabetes and deafness.
Fundus autofluorescence is a non-invasive imaging modality that can map naturally and pathologically occurring fluorophores in the posterior pole, first utilized for in vivo fundus imaging in 1995 characterizing the intrinsic autofluorescent properties of the retina. Technical improvements have developed in the interim utilizing the fluorescent properties of lipofuscin within the retinal pigment epithelium (RPE) to create an image. Lipofuscin, a byproduct of lysosomal breakdown of photoreceptor outer segments, is composed of numerous bisretinoids including A2E, A2PE, isoA2E, and A2-DHP-PE. When subjected to a light source, these bisretinoids absorb blue light with a peak excitation wavelength of 470nm and emit yellow-green light with a peak wavelength of 600nm, depending on the chemical makeup of the lipofuscin. Since many retinal pathologies often lead to RPE dysfunction and an accumulation of lipofuscin, abnormal patterns of autofluorescence (AF) on FAF imaging can act as markers for retinal disease.
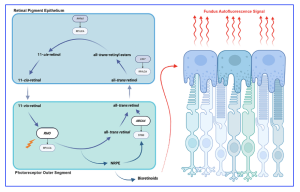
Figure 1. Retinoid visual cycle and the origin of fundus autofluorescence signal. An illustration of the retinoid visual cycle that takes place between the retinal pigment epithelium and the outer segments of photoreceptors is shown. Interruption of the visual cycle at any point can lead to the development of inherited retinal degenerations. Accumulation of bisretinoids in retinal pigment epithelium cells as a byproduct of the cycle is the source of fundus autofluorescence signal. [The research work is licensed under the terms of the Creative Commons Attribution-Non Commercial-No Derivatives License 4.0 (CCBY-NC-ND), cited by Oh et al., entitled by: “Fundus Autofluorescence in Inherited Retinal Disease: A Review”, Cells 2025, 14, 1092].
The researchers explained that FAF is “an indispensable tool in the diagnosis and monitoring of numerous retinal diseases. Across the spectrum of IRDs, including Stargardt disease and pattern dystrophies, RP, choroideremia, and mitochondrial retinopathies, FAF has demonstrated consistent utility in disease diagnosis, monitoring of progression, and guiding prognostication. Several biomarkers for potential clinical trials, such as changes in hyperautofluorescent ring size or area of hypoautofluorescent atrophy, are increasingly being considered as potential outcome measurements in clinical trials. As our understanding of IRDs deepens, FAF will likely remain a mainstay of both clinical care and research.”
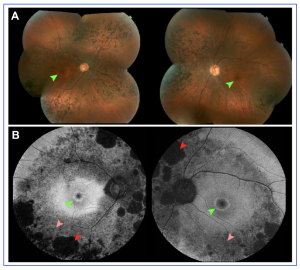
Figure 2. Hyperautofluorescent rings in retinitis pigmentosa seen on fundus autofluorescence compared to color fundus photography. (A) Montage color fundus photographs of a 36-year-old patient with autosomal dominant retinitis pigmentosa due to mutations in RHO demonstrate waxy pallor of the optic nerve, vascular attenuation and nasal and inferior bone spicules. (B) 55◦ field fundus autofluorescence demonstrates a central hyperautofluorescent ring, smaller in the right eye than the left, which is not seen on color fundus photographs (green arrows). Areas of both definitely decreased autofluorescence (red arrows) and questionably decreased autofluorescence can be seen (pink arrows). [The research work is licensed under the terms of the Creative Commons Attribution-Non Commercial-No Derivatives License 4.0 (CCBY-NC-ND), cited by Oh et al., entitled by: “Fundus Autofluorescence in Inherited Retinal Disease: A Review”, Cells 2025, 14, 1092].
In addition, fundus autofluorescence provides a quantitative outcome measure for natural history studies, valuable for subsequently supporting clinical trials from new experimental treatments. The recent US researchers have commented that “such studies have become increasingly common, particularly for genetically defined forms of RP, with many incorporating FAF to monitor disease progression. In the past two years alone, multiple natural history studies for various forms of RP have been described”. According to the researchers, FAF enables objective quantitative assessment of retinal atrophy to evaluate whether treatment slows disease progression compared to untreated eyes”.