Researchers at the Biomedical Graduate Program and the Department of Ophthalmology, Mayo Clinic, Rochester, Minnesota, USA, have published a systematic review on serious adverse events (SAEs) associated with retinal viral gene therapy, and examining trends influencing SAE occurrences in human gene therapy surgeries and pre-clinical animal trials. The study showed that subretinal injections had higher efficacy than intravitreal injections, and showed that subretinal injections were associated with more serious adverse events, compared to intravitreal injections. The researchers concluded that there is a need for optimised delivery methods, refined dosing protocols, and improved post-treatment monitoring to improve safety and effectiveness in gene therapy for retinal degenerations.
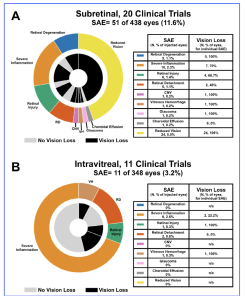
The researchers evaluated SAEs from trial results from inherited retinal disease (IRDs), with the prevalence of all IRDs being 1:1380, and with the most common being retinitis pigmentosa (RP, 1:4,000), Stargardt disease (1:10,000), and choroideremia (CHM, 1:50,000). The most commonly utilised viral vector in retinal diseases is adeno-associated virus type 2 (AAV2) across different serotypes, such as AAV2/2 (RPE tropism) and AAV2/8 (cone photoreceptor tropism) and the three main methods of delivery are intravitreal, subretinal, or suprachoroidal injections. Following a systematic review process, the researchers collected 31 clinical trial studies and SAEs were recorded in 51 out of 438 eyes (11.6%) that received subretinal injections and in 11 out of 348 eyes (3.2%) that received intravitreal injections. There were fewer intravitreal-related SAEs and less vision loss compared to subretinal gene therapy.
Figure 1. Serious adverse event breakdown by injection route. For subretinal trials (A) and intravitreal trials (B), the outer wheels show the proportion of SAE type, while the inner wheel shows the proportion of eyes that experienced vision loss due to the associated SAE. For all injected eyes, the number and percentage of eyes that experienced each SAE are included in the provided table (left columns). For any given SAE, the number and percentage of eyes experiencing vision loss are included in the provided tables (right columns).
[The research work is licensed under the terms of the Creative Commons Attribution-Non Commercial-No Derivatives License 4.0 (CCBY-NC-ND), cited by Berger et al., entitled by: “Retinal Viral Gene Therapy: Impact of Route of Administration on Serious Adverse Events—A Systematic Review”, Clinical & Experimental Ophthalmology, 2025; 0:1–19 https://doi.org/10.1111/ceo.14593].
Inflammation was the predominant SAE following intravitreal injections, whereas unexplained vision loss was the most common for subretinal injections. Clinical trials utilising subretinal injections met primary or secondary efficacy endpoints more than intravitreal trials. Eighteen studies (429 eyes) of post-approval LUXTURNA (voretigene neparvovec) were reviewed, and SAEs were reported in 24.7% of eyes, retinal degeneration being most common (20.7%). For 58 animal studies, SAEs were recorded in 17.3% of eyes that received subretinal injections and 8.7% of eyes that received intravitreal injections. In addition, the researchers commented that the purity and quality of drug production is crucial, in particular to the number of capsids loaded with viral genomes. Some capsids may be empty and some may be full. Without data being made available on the production process, it will be unclear on what the level of dosage of the gene therapy treatment. In conclusion, the researchers stated that, “we recommend that future viral gene therapy studies evaluate chorioretinal atrophy (CRA) specifically as an adverse event. Studies should follow their subjects for an extended period, preferably at least a year, to assess the development of CRA. For consistency, viral genomes and capsid particles should both be reported. Additionally, when reporting inflammation, quantitative methods should be used such as Standardisation of Uveitis Nomenclature (SUN). For animal studies, visual function assessments should be included in safety assessments. It is also recommended to use animal disease models, when possible, to accurately assess safety.”