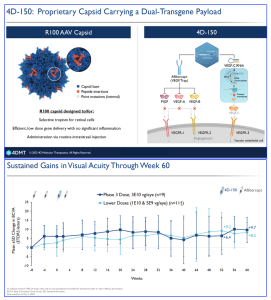
4D Molecular Therapeutics (Nasdaq: FDMT, 4DMT), based in Emeryville, California, have announced 60-week summary data from their “SPECTRA” diabetic macular edema (DME) Phase III clinical trial. 4D-150 is an intravitreal gene therapy treatment expressing both aflibercept and a VEGF-C inhibitory RNAi, proposing a dual-transgene to inhibit four members of the VEGF angiogenic family targeting DME (VEGF A, B, C and PlGF). According to the company, 4D-150 appears to be well tolerated with no intraocular inflammation with sustained gains in visual acuity and anatomic control. The Phase 3 dose (3×10-1 vg/eye) have reported a 78% reduction in treatment burden vs. projected on-label aflibercept 2mg Q8W.
4D-150 is an investigational agent designed to provide sustained delivery of anti-VEGF (aflibercept and anti-VEGF-C) utilizing thecompany’s customized intravitreal vector, R100. 4D-150 is being developed for wet AMD and DME for the purpose of avoidpatients from burdensome injections while preserving vision. The company’s product candidates are in clinical or preclinical development, not yet been approved for marketing by regulatory authorities. The company is aimed to align a single Phase 3 clinical trial for both the FDA and EMA, based on data generated for 4D-150 from two planned Phase 3 clinical trials in ta “4FRONT” wet age-related macular degeneration (wet AMD) program, potentially acceptable as the basis for a marketing authorization application (MAA) submission for 4D-150 in DME.
In the SPECTRA (DME) study, the company reported efficacy results through 60 Weeks from a Phase 3 dose showing a sustained gain of best corrected visual acuity (BCVA) of +9.7 letters and a sustained improvement in anatomic control, with reduction of CST, as measured by optical coherence tomography (OCT), of -174 µm. Following the announcement, David Kirn, M.D., Co-founder and Chief Executive Officer of 4DMT stated that, “the consistency of dose response, safety and efficacy data we’ve seen with 4D-150 across all patients evaluated in both DME and wet AMD reinforces our belief that 4D-150 has the potential to become a true backbone therapy, and may significantly improve the lives of millions of patients living with retinal vascular disease. With alignment from both FDA and EMA for a single Phase 3 DME trial to complement the 4FRONT wet AMD program, we believe we have a clear streamlined path to bring 4D-150 to patients with high unmet need”.