Atsena Therapeutics, based in Durham, North Carolina, has announced that the U.S. Food and Drug Administration (FDA) has agreed to the expansion of the company’s ongoing Phase 1 / 2 (“LIGHTHOUSE study”), of ATSN-201, into a continuous Phase 1 / 2 / 3 trial, enabling it to serve as a pivotal trial to support a Biologics License Application (BLA) for the treatment of X-linked retinoschisis (XLRS). There are no approved treatments for XLRS, which is typically diagnosed in early childhood and affects ~30,000 males in the U.S. and the EU. The ATSN-201 treatment uses a novel “spreading capsid” technique to achieve therapeutic levels of gene expression in photoreceptors of the central retina, while avoiding the surgical risks of foveal detachment. Previously, the company received “Fast Track” designation, granted by the FDA for treatments intended to address serious or life-threatening diseases that have demonstrated the potential to meet an unmet medical need. The company has additionally received Regenerative Medicine Advanced Therapy (RMAT) and Orphan Drug Designations from the FDA. ASTN-201 (rAAV.SPR-hGRK1-hRS1syn) is a subretinal gene therapy product being developed to introduce the functional human retinoschisin (hRS1) gene to photoreceptors.
X-linked retinoschisis (XLRS) is an uncommon inherited retinal degeneration (IRD), with a prevalent estimate ranging from 1 to 5,000 to 1 in 25,000 cases, mainly affected in males however, a number of female carriers may exhibit the phenotype due to altered X-inactivation. XLRS is caused by mutations in the RS1 gene, encoding a retinal protein, retinoschisin, which plays a key role in maintaining the retinal structure. The protein is expressed and localized in the surface of photoreceptor and bipolar cells, functioning in cell-to-cell adhesion, maintaining tissue integrity and homeostasis, and regulates apoptosis signalling. The deficiency or absence of retinoschisin in XLRS leads to photoreceptor degeneration due to cell apoptosis and disruption of the retinal layers. The disorder causes schisis (or splitting) of the neural retina leading to reduced visual acuity and disturbances in central vision, strabismus or nystagmus, affecting from an early age impacting daily function, including difficulty with reading and obtaining a driver’s license. Mutations of the RS1 gene comprise >200 alterations, most of which are missense and the majority occur in exon 4-6, corresponding to the discoidin domain of the protein. The disease can be highly variable make it challenging to correlate genotype-to-phenotype for patients.
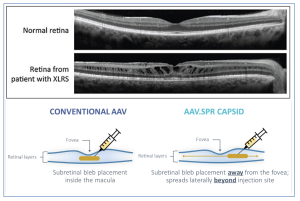
Figure 1. Highlighted at Atsena Therapeutics’ website*, the company shows to schisis (or splitting) of the neural retina leading to reduced visual acuity caused by mutations in the RS1 gene, and; delivery optimization of AAV.-RS1.SPR (spread) allowing for subretinal injection in the peripheral retina, avoiding foveal detachment and potential trauma and efficiently transduces the vector to foveal photoreceptors covering a larger area resulting in wider area of retinal transduction.(* https://atsenatx.com/wp-content/uploads/2023/12/ESGCT2023_INDXLRS-1.pdf).
Following the announcement, Patrick Ritschel, Chief Executive Officer of Atsena Therapeutics commented that, “this regulatory milestone marks another significant step toward delivering a potentially first- and best-in-class gene therapy for patients living with XLRS. The agency’s agreement will expedite the clinical development of ATSN-201 by at least 1.5 years, compared to a separate Phase 3 clinical trial. If approved, this would be the first available treatment for XLRS, offering hope to patients and families affected by this inherited retinal disease. We’re grateful for the FDA’s continued guidance as we continue to advance this trial and prepare for filing a BLA in early 2028.”