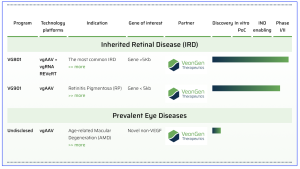
VeonGen Therapeutics GmbH, based in Munich, Germany, have announced the progression of two proprietary gene therapies to the clinic based on a novel gene therapy platform, and the re-branding of the company, formerly known as ViGeneron. The re-branding was aimed to re-position their company from a pre-clinical to a clinical-stage company for patients with high unmet medical needs, and progressing lead programmes of “VG801”, for Stargardt’s disease, and “VG901” for retinitis pigmentosa. VG801 is a novel dual AAV gene therapy for Stargardt disease and other ABCA4 related retinal disorders, and VG901 is an intravitreally delivered AAV gene therapy for retinitis pigmentosa caused by CNGA1 mutations. Commenting on their announcement, Dr. Caroline Man Xu, Co-founder & Chief Executive Officer of VeonGen Therapeutics stated that, “this rebranding reflects our journey – from a platform innovator to a clinical -stage company with two gene therapies in the clinic. With VG801 and VG901 progressing in clinical trials and our platforms demonstrating robust translational potential, we are well positioned to expand the frontier of genetic medicine in ophthalmology and beyond.”
VeonGen presents three differentiated technology platforms: (1) engineered AAV capsids with enhanced transduction efficiency and the ability to overcome biological barriers, enabling less invasive delivery routes such as intravitreal and systemic administration; (2) a trans-splicing platform – “vgRNA REVeRT (Reconstitution via mRNA Trans-splicing)” – designed to enable the delivery and reconstitution “of large genes exceeding the 4.7 kb AAV cargo limit at the mRNA level in tissues targeted by a selected AAV capsid, and finally; (3) a third platform, “AAV Transactivation”, using CRISPR/Cas technology enabling the activation or repression of disease relevant genes using gene supplementation or applying novel applications in genetic diagnostics.
Figure 1. The company’s pipeline is presented online, available at https://veongen.com/pipeline/
According to the company, VG801 is a novel mRNA trans-splicing gene therapy using dual adeno-associated virus (AAV) vectors to deliver the full-length human native ABCA4 gene. Their gene supplementation treatment aims to address biallelic ABCA4 mutation-associated Stargardt disease, and other ABCA4-linked retinal dystrophies. VG801 has previously demonstrated in vivo functionality and expression of the full-length ABCA4 gene in an animal model of Stargardt disease, as well as efficient, expression and safety in non-human primate retinas. The company’s lead program VG801, has received FDA Rare Pediatric Disease Designation and is now progressing in a first-in-human Phase 1/2 trial for Stargardt disease, with functional endpoint development underway through the FDA’s RDEA program. Finally, VG901, to treat patients with retinitis pigmentosa (RP) caused by mutations in the CNGA1 gene have received FDA Rare Pediatric Disease Designation (RPDD) to VG901, and the company’s independent Data Safety Monitoring Board (DSMB) has previously approved a dose escalation study in an ongoing Phase 1b clinical trial. Mutations in the CNGA1 gene, encoding a subunit of CNG channels in rod photoreceptors, are reported to cause approximately 2% – 8% of autosomal recessive retinitis pigmentosa (arRP).
The company’s key personnel are available at the VeonGen’s website and can provide further details below (https://veongen.com/about/#our-core-team ):