Clinical researchers at the Department of Ophthalmology and the Vision Centre of Excellence, Akron Children’s Hospital, Ohio, have reported a molecular diagnostic rate of 41.5% from pediatric patients with infantile nystagmus syndrome (INS) using next-generation sequencing (NGS) with targeted gene panels to detect pathogenic variants. Their study achieved a definitive diagnosis in 85 of 205 patients and a total of total of 83 pathogenic and likely pathogenic variants identified across 30 genes. The research team reports the work as being the largest genetically characterized INS cohort from a single vision centre. The US team stated that their “study underscores the utility of NGS in diagnosing INS-associated inherited ocular diseases, providing essential insights for targeted interventions and identifying patients as candidates potentially eligible for ongoing gene-based therapy clinical trials.”
Infantile nystagmus syndrome (INS) is an ocular motor disorder characterized by involuntary, rhythmic oscillation of the eyes, often manifesting within the first six months and is the most common form of nystagmus in infancy and childhood. The researchers showed that ninety per cent of INS cases are associated with inherited ocular disorders with distinct inheritance patterns, including autosomal recessive, autosomal dominant, X-linked, and mitochondrial modes. Obtaining of a genetic diagnosis for INS patients is critical for several aspects including genetic counselling, support of family members, and importantly, for defining eligibility for up-coming gene-based therapies.
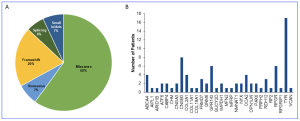
Following their study, clinical researchers enrolled 205 unrelated pediatric patients who underwent genetic testing, 117 males (57%) and 88 females (43%). Ages at genetic testing ranged from 0.3 to 40 years, with a mean of 8.54 ± 7.91 years and the most frequently mutated genes included TYR (n = 17 [20%]) and OCA2 (n = 4 [4.7%]) for oculocutaneous albinism, CNGB3 (n = 8 [9.4%]) for achromatopsia, GPR143 (n = 6 [7%]) for X-linked ocular albinism, RPGR (n = 6 [7%]) for X-linked retinitis pigmentosa, ABCA4 (n = 5 [5.9%]) for Stargardt disease, and FRMD7 (n = 3 [3.5%]) for idiopathic INS. Their study found that eight LCA-associated genes (AIPL1, CABP4, GUCY2D, IMPDH1, NMNAT1, RDH12, PRPH2 and RPGRIP1) accounted for 15% of genetically diagnosed cases, with other genes having lower prevalence.
Figure 1. Types of gene variants and prevalence of disease-associated causative genes identified in the genetically solved INS cohort. (A) Pie chart showing the distribution of all the gene variants underlying INS or its associated conditions according to their types. (B) Prevalence of disease-associated genes harboring causative variants in patients with INS. [This graphic and work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, authored Gong, X., et al. Clinical spectrum and molecular characteristics of inherited ocular diseases in a cohort of pediatric patients with infantile nystagmus syndrome. Invest Ophthalmol Vis Sci. 2025;66(4):39. https://doi.org/10.1167/iovs.66.4.39.]
Accurate interpretation of genetic variants is crucial for precise diagnosis, clinical management, genetic counselling, and potential access to gene-based therapies due to the prevalence of nystagmus across several ocular disorders. INS-associated IODs exhibit phenotypic heterogeneity due to diverse genotypes. Clinically, they can be classified into two main categories: non-syndromic and syndromic. Non-syndromic IODs primarily result from mutations in retina-specific genes, including those associated with achromatopsia, LCA, X-linked RP, blue cone monochromatism and congenital stationary night blindness (CSNB). Syndromic IODs involve multiple organs beyond the eyes, such as albinism. In concluding their results, the researchers commented that the molecular diagnosis in 41.5% of pediatric INS cases, using targeted NGS, had “actionable findings for gene-based therapies in 35% of solved cases. The remaining unresolved cases highlight the need for more comprehensive genetic testing, including WGS, and further research into non-coding regions and CNVs. A multidisciplinary approach, integrating clinical, genetic, and imaging data, is essential for managing INS and its associated conditions.”