Ashvattha Therapeutics, based in Redwood City, California, have announced positive interim Phase 2 results for migaldendranib (MGB) and hydroxyl dendrimer, aimed to treat retinal vascular diseases. The company are focused on a new class of clinical-stage nanomedicine therapeutics targeting regions of inflammation. MGB is a VEGF receptor tyrosine kinase inhibitor delivered via a hydroxyl dendrimer platform, aimed to selectively targets activated cells in the retina, thereby reducing VEGF expression and fluid production in both eyes simultaneously. Results for their experimental drug treating AMD and DME, presented data at the Association for Research in Vision and Ophthalmology (ARVO) Annual Meeting, (May 4 – May 8, 2025, in Salt Lake City, UT).
The migaldendranib and dendrimer is aimed to block forms of vascular endothelial growth factor (VEGF), expected to clear the kidney and liver avoiding toxicity. The delivery uptake is only targeted to the retinal pigment epithelial (RPE) cells and macrophages in actively inflammatory lesions and is retained in the cells for 30 days and then cleared from the body in ~2 days.
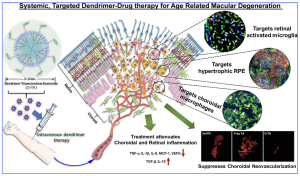
A graphical abstract of the innovation, using a triamcinolone acetonide (TA) conjugate, shows a systemic hydroxyl-terminated polyamidoamine dendrimer-TA conjugate (D-TA) is selectively taken up by the injured mi/ma and RPE (without the need for targeting ligands). D-TA suppresses choroidal neovascularization significantly (by >80%, >50-fold better than free drug), attenuates inflammation in the choroid and retina, by limiting macrophage infiltration in the pathological area, significantly suppressing pro-inflammatory cytokines and pro-angiogenic factors, with minimal side effects to healthy ocular tissue and other organs. [Authored by Kambhampati, Siva P. et al., Systemic dendrimer nanotherapies for targeted suppression of choroidal inflammation and neovascularization in age-related macular degeneration. Journal of Controlled Release, Volume 335, 10 July 2021, Pages 527-540, also listed at Ashvattha Therapeutics website, https://avttx.com/resources/?_sft_resource_type=publications].
Following a Phase 2 open-label study of subcutaneous (subQ) MGB, 27 subjects (16 with wet AMD [age-related macular degeneration] and 11 with DME [diabetic macular edema]) were previously treated with anti-VEGF intravitreal injections (IVT). Subjects received a single anti-VEGF IVT and subQ MGB at baseline, followed by subQ MGB every 2 or 4 weeks for 40 weeks. The interim analysis focuses on 24-week data from 8 wet AMD and 6 DME subjects. Following of completing 24 weeks of treatment, summary results presented at ARVO showed:
- 9% reduction in wet AMD subjects and 80.0% reduction in DME subjects compared to the 24 weeks prior to Day 1, representing a 5.0-fold decrease in anti-VEGF treatment burden for both indications
- Bilateral treatment effect with 66.7% reduction in fellow eye IVT injections for wet AMD subjects and 85.8% reduction for DME subjects
- Mean best corrected visual acuity improved by 3 letters in study eye of wet AMD subjects (from baseline to week 24) and improved by 4.5 letters in study eye of DME subjects (from baseline to week 24)
- Mean central subfield thickness improved by 45.5 microns in wet AMD subjects and by 69.1 microns in DME subjects
- No serious adverse events or ocular adverse events related to MGB were reported. Injection site reactions were reported in 5 subjects (3 wet AMD, 2 DME), with only 26 reactions across 302 total subcutaneous injections (8.6%), mostly Grade 1 (mild)
Following their presentation at ARVO, Jeff Cleland, Ph.D., CEO of Ashvattha Therapeutics, stated that, “these updated Phase 2 results demonstrate MGB’s potential to address a significant unmet need in retinal vascular diseases. A monthly subcutaneous injection that effectively treats both eyes simultaneously while improving vision and reducing retinal fluid addresses a significant unmet need. We look forward to advancing this promising therapy into late-stage clinical development.”