A consortium of clinical researchers and trial experts have launched a new updated statement published by 39 researchers*, led by Professor Sally Hopewell, based in the Oxford Clinical Trials Research Unit, Centre for Statistics in Medicine, University of Oxford, UK. The “CONSORT 2025 statement” (Consolidated Standards of Reporting Trials) comprises a 30-item checklist providing a minimum set of items to be included in a report of a randomized trial. The CONSORT 2025 statement provides an invaluable resource for either new or seasoned clinicians how to optimally conduct high-quality research on randomised trials, presenting a backbone or scaffold to ensure their study benefits the patient community, grounded in evidence-based medicine. The statement outlined that, “the objective of the CONSORT 2025 statement is to provide a minimum set of recommendations to authors about the content they should include in order to report their trials in a clear, complete, and transparent manner9,10. Readers, peer reviewers, clinicians, guideline writers, patients and the public, and editors can also use CONSORT 2025 to help them appraise the reporting of randomized trials.”
The Consolidated Standards of Reporting Trials (CONSORT) are a set of guidelines adopted by various journals regarding the content of published reports of randomised trials. The guidelines arose from researchers in the 1990s to ensure that improvements and consistency applied for randomized trials were to be used across all researchers internationally and these initiatives were originally published in 1996 and then revised in 2001 and 2010. The new revision is now published in 2025, issued at https://www.consort-spirit.org, and simultaneously published in BMJ, JAMA, The Lancet, Nature Medicine and PLoS Medicine(see BMJ. 2025; 388:e081123. https://dx.doi.org/10.1136/bmj-2024-081123).
The summary points for the new CONSORT 2025 statement were outlined in the consortium’s work including:
- To interpret a randomised trial accurately, readers need complete and transparent information on its methods and findings.
- The CONSORT 2025 statement provides updated guidance for reporting the results of randomised trials, that reflects methodological advancements and feedback from end users.
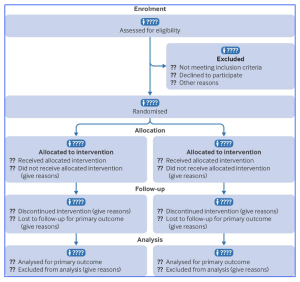
- The CONSORT 2025 statement consists of a 30-item checklist of essential items, a diagram for documenting the flow of participants through the trial, and an expanded checklist that details the critical elements of each checklist item.
- Authors, editors, reviewers, and other potential users should use CONSORT 2025 when writing and evaluating manuscripts of randomised trials to ensure that trial reports are clear and transparent.
The new CONSORT 2025 statement has updated the checklist, up from 25 items in 2010, now providing 30 items on the list, including “seven new checklist items, revised three items, deleted one item, and integrated several items from key CONSORT extensions (Harms, Outcomes and Non-Pharmacological Treatment). The CONSORT 2025 statement supersedes the CONSORT 2010 statement, which should no longer be used. Journal editors and publishers should update their instructions to authors to refer to CONSORT 2025.”
Figure 1. CONSORT 2025 flow diagram. Flow diagram of the progress through the phases of a randomised trial of two groups (ie, enrolment, intervention allocation, follow-up, and data analysis). CONSORT=Consolidated Standards of Reporting Trials. [This figure is provided from an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license: http://creativecommons.org/licenses/by/4.0/, authored by Hopewell, S et al., entitled, “CONSORT 2025 statement: updated guideline for reporting randomised trials”, published in BMJ 2025; 389 doi: https://doi.org/10.1136/bmj-2024-081123].
According to the researchers in their publication the authors commented that: “Extensions to CONSORT have been developed to tackle the methodological issues associated with reporting different types of trial designs, data and interventions. Examples of extensions for trial designs include recommendations for adaptive designs, cluster trials, crossover trials, early phase trials, factorial trials, non-inferiority and equivalence trials, pragmatic trials, multi-arm trials, n-of-1 trials, pilot and feasibility trials, and within-person trials.” In summary, the authors concluded that, “we also strongly recommend the submission of a completed CONSORT 2025 checklist as part of the manuscript submission process, detailing where in the manuscript checklist items are reported, and uploaded as part of the supplementary materials. An explicit description of what was done and what was found, without ambiguity or omission, best serves the interests of all readers.”
*
- Oxford Clinical Trials Research Unit, Centre for Statistics in Medicine, University of Oxford, UK.
- Department of Medicine, Women’s College Research Institute, University of Toronto, Toronto, Ontario, Canada.
- UK EQUATOR Centre, Centre for Statistics in Medicine, University of Oxford, Oxford, UK.
- Centre for Evidence-Based Medicine Odense and Cochrane Denmark, Department of Clinical Research, University of Southern Denmark, Odense, Denmark.
- Open Patient data Explorative Network, Odense University Hospital, Odense, Denmark.
- Centre for Journalology, Clinical Epidemiology Programme, Ottawa Hospital Research Institute, Ottawa, Ontario, Canada.
- Department of Obstetrics and Gynecology, School of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
- Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India.
- Office of Science Dissemination, Centers for Disease Control and Prevention, Atlanta, GA, USA.
- Department of Biostatistics and Epidemiology, School of Public Health, Center for Pharmacoepidemiology and Treatment Science, Rutgers University, New Brunswick, NJ, USA.
- JAMA Network Open, Chicago, IL, USA.
- Centre for Health Research and Development, Society for Applied Studies, New Delhi, India.
- Child Health Evaluation Services, The Hospital for Sick Children Research Institute, Toronto, Ontario, Canada.
- Department of Psychiatry, University of Toronto, Toronto, Ontario, Canada.
- Aberdeen Centre for Evaluation, University of Aberdeen, Aberdeen, UK.
- Project PINK BLUE – Health & Psychological Trust Centre, Utako, Abuja, Nigeria.
- Department of Sociology and Gerontology, Miami University, Oxford, OH, USA.
- Department of Medical Statistics, London School of Hygiene and Tropical Medicine, London, UK.
- Nuffield Department of Primary Care Health Sciences, University of Oxford, Oxford, UK.
- Ottawa Hospital Research Institute, Ottawa, Ontario, Canada.
- Department of Medicine, Northwestern University Feinberg School of Medicine, Chicago, IL, USA.
- Department of Epidemiology and Population Health, Stanford University, Palo Alto, CA, USA.
- Institute for Evidence-Based Healthcare, Faculty of Health Sciences and Medicine, Bond University, Robina, Queensland, Australia.
- Departments of Medicine, of Epidemiology and Population Health, of Biomedical Data Science, and of Statistics, and Meta-Research Innovation Center at Stanford (METRICS), Stanford University, Stanford, CA, USA.
- MRC Clinical Trials Unit at University College London, London, UK.
- University College London, UCL Great Ormond Street Institute of Child Health, London, UK.
- NIHR Exeter Biomedical Research Centre, Faculty of Health and Life Sciences, University of Exeter, Exeter, UK.
- Edinburgh Clinical Trials Unit, Usher Institute-University of Edinburgh, Edinburgh BioQuarter, Edinburgh, UK.
- The BMJ, BMA House, London, UK.
- Harvard Medical School, Boston, MA, USA.
- Université Paris Cité, Inserm, INRAE, Centre de Recherche Epidémiologie et Statistiques, Université Paris Cité, Paris, France.
- Clinical Trials Ontario, MaRS Centre, Toronto, Ontario, Canada.
- Duke Clinical Research Institute, Duke University Medical Center, Durham, NC, USA.
- Department of Emergency Medicine, University of California, Los Angeles, CA, USA.
- South African Medical Research Council, Cape Town, South Africa.
- Warwick Applied Health, Warwick Medical School, University of Warwick, Coventry, UK.
- MRC/CSO Social and Public Health Sciences Unit & Robertson Centre for Biostatistics, Institute of Health and Wellbeing, University of Glasgow, Glasgow, UK.
- Department of Health Research Methods Evidence and Impact, McMaster University, Hamilton, Ontario, Canada.
- St Joseph’s Healthcare Hamilton, Hamilton, Ontario, Canada.
- York Trials Unit, Department of Health Sciences, University of York, York, UK.
- Faculty of Medicine and Dentistry, University of Alberta, Edmonton, Alberta, Canada.
- Université Paris Cité and Université Sorbonne Paris Nord, Inserm, INRAE, Centre for Research in Epidemiology and Statistics (CRESS), Paris, France.
- Centre d’Epidémiologie Clinique, Hôpital Hôtel Dieu,AP-HP, Paris, France.