EURETINA Congress, Barcelona – Sept. 20th, 2024: Prof. M. Dominick Fischer, based at Nuffield Laboratory of Ophthalmology, University of Oxford and the Oxford Eye Hospital, NHS Foundation Trust, has presented new 4-year interim results from a real-world study on the treatment of voretigene neparvovec (VN, AAV2-hRPE65v2) for RPE65 mutation-associated IRD patients. The study provides an ongoing, prospective, longitudinal, multi-centre, multinational (ex-US), observational, registry-based protocol, predicted to complete in 2029. EURETINA members were told that 4-year interim results showed that treatment provides safety and effectiveness and that the results to date are consistent with the safety profile of VN, and that the majority of patients receive sustained improvements in visual function.
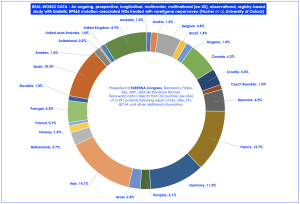
VN represents the first licensed treatment for patients with vision loss due to a specific inherited retinal degeneration (IRD), caused by confirmed biallelic RPE65 mutations by both the FDA and EMA, now applied to hundreds of patients worldwide. The course of treatment comprises a single injection into each eye, understood with no further intervention required. The real world study, on behalf of the “PERCEIVE” Study group, and fully funded and sponsored by Novartis Pharma, have collected demographics, medical records, PROs (patient reported outcomes), CROs (caregiver reported outcomes), VN surgical administration, pregnancy outcomes and ophthalmic examination data, including FST, BCVA, VF and OCT. A total of n=291 patients were followed across 24 countries, with the largest cohorts followed in Italy (14.1%), France (12.7%), Germany (11.3%), Spain (10.3%) and the United Kingdom (8.9%), as summarised below:
Figure 1: Real-world data presented by Prof. M. Dominik Fischer at EURETINA Congress, Barcelona, Sept. 20th, 2024; summary breakdown of real-world data across 24 countries with a n=291 patients.
In terms of ocular AEs and AESIs (adverse events of special interest), the presentation of the interim results reported that 186 patients (63.9%) had one or more ocular AEs, with the top three AEs found in retinal degeneration (77 (26.5%)), IOP increase (49, 16.8%) and injection site atrophy (19 (6.5%)). In respect of AESIs, 167 patients (57.4%) had one or more ocular AESIs and their top three AESIs found were chorioretinal atrophy (CRA) (86 (29.6%)), IOP increase (55, 18.9%) and intraocular inflammation and / or infection related to procedure (37 (12.7%)). From earlier reports on a previous PERCEIVE study showed that CRA was identified, “as a new adverse drug reaction, which was not described in pivotal VN trials as an adverse event”, and on the current 4-year interim results, CRA showed 86 patients (29.6%) for this AESI was based on “investigator judgement” when analysing the data. Of patients with CRA, 58.1% were female (n=50), and 98 eyes (74.8%) had at least one event at the injection site. In terms of evaluating the CRA and grouping the events the author listed retinal degeneration, injection site atrophy, retinal depigmentation, retinal dystrophy, injection site discolouration and macular degeneration.
Finally, in regards to efficacy of VN, delegates learned that the real-world data showed that there was sustained FST improvement observed up to years 4 years however, there was no clinically meaningful change of mean BCVA from baseline over this period
Figure 2: Interim results presented by Prof. M. Dominik Fischer at EURETINA Congress, Barcelona, Sept. 20th, 2024; real-world data on FST (while light) and BCVA outcomes measured over 4-years..
In conclusion, Prof. Fischer told delegates that 4 interim results showed that treatment provides safety and effectiveness and this was consistent with the safety profile of VN and that the majority of patients showed sustained improvements in visual function.