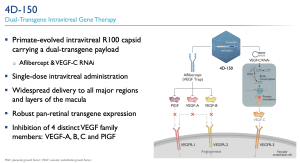
4D Molecular Therapeutics (Nasdaq: FDMT, 4DMT), based in Emeryville, California, have announced interim 24-week data from their “PRISM” (WET AMD) and “SPECTRA” (DME) phase I/II clinical trials evaluating the intravitreal “4D-150”, an experimental treatment for a broad neovascular AMD patient population. 4D-150 is an intravitreal gene therapy treatment expressing both aflibercept and a VEGF-C inhibitory RNAi, proposing a dual-transgene to inhibit four members of the VEGF angiogenic family targeting wet AMD and DME (VEGF A, B, C and PlGF). According to the company, 4D-150 is designed for single, low-dose intravitreal delivery for transgene expression from the retina without significant inflammation.
The clinical trial, listed on clinicaltrials.gov (NCT05197270), has a formal title of, “A Phase 1/2 Dose-Escalation and Randomized, Controlled, Masked Expansion Trial of Intravitreal 4D-150 Gene Therapy in Adults With Neovascular (Wet) Age-Related Macular Degeneration”. The randomised trial uses an open-label, 3+3 dose escalation design with their AAV- gene therapy experimental treatment of miRNA targeting VEGF-C and a codon-optimized sequence encoding aflibercept. Their primary outcome measure was the incidence and severity of treatment emergent adverse events (TEAEs) and serious adverse events (SAEs), including clinically significant changes in safety parameters over 1 year. Secondary outcome measures included the time to receiving the first supplemental aflibercept injection; % of patients requiring supplemental aflibercept injections over 52 weeks; number of supplemental aflibercept injections over 1 year; BCVA using ETDRS visual acuity chart, and; the change of central subfield thickness (CST) over time 1 year (measured by spectral domain optical coherence tomography (SD-OCT)).
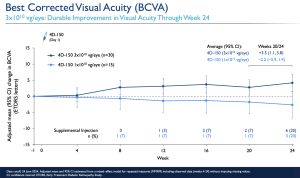
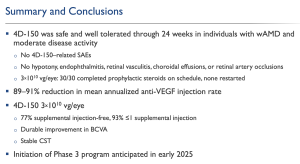
In summary, the company’s top-line results suggested an improvement in mean BCVA, from baseline through week 24, of +4.2 letters, having applied 3E10 vg/eye dose. The company reported that 139 patients were treated to date, with 4D-150 across wet age-related macular degeneration (wet AMD; PRISM trial) and diabetic macular edema (DME; SPECTRA trial). In addition, there was no significant inflammation, hypotony, retinal vasculitis, choroidal effusions or retinal artery occlusions with up to 2.5 years of follow-up in 51 patients treated to date with 3E10 vg/eye dose, and with a topical corticosteroid regimen.
On the company’ public website (https://4dmoleculartherapeutics.com), their interim data proposed summary information including:
According to Arshad M. Khanani, M.D., M.A., FASRS, Director of Clinical Research at Sierra Eye Associates and Clinical Professor at University of Nevada, Reno, following the announcement of the interim data, stated, “patients suffering from wet AMD face a substantial treatment burden, requiring frequent intravitreal injections throughout their lives, which significantly impacts quality of life not only for the patients themselves but also for their families and caregivers. The PRISM Phase 2 data at 24 weeks across multiple populations, and long-term data collected over two and a half years, confirms 4D-150’s potential to significantly reduce the treatment burden and maintain vision through a safe, single intravitreal injection. I look forward to contributing to the Phase 3 trial, and to further advancing this treatment option for individuals with wet AMD.”