In a recent study published in the prestigious journal Nature Communications (2024 Jul 21;15[1]:6150), researchers have reported that an upstream regulator, “AKT2” (a member of the subfamily of serine/threonine kinases), appears to act with a central role in regulation of lysosomal function and autophagy, critically engaged in dry-AMD pathology and drusen. The researchers, based in the Department of Ophthalmology, University of Pittsburgh School of Medicine, and the Wilmer Eye Institute, The Johns Hopkins University School of Medicine, Baltimore, has shown that a knock-in genetic model appears to reproduce a dry-AMD-like phenotype, potentially capable to target a therapeutic intervention to address dry-AMD. To provide relevance to AMD in humans, researchers showed that, “AKT2 signalling is increased to impair lysosomal function in the RPE of AMD donor eyes and iPSC derived RPE cells that harbour the high-risk CFH 402H variant”. The researchers have commented that while, “the underlying mechanism of lysosomal dysfunction in CFH Y402H harbouring RPE cells has been unknown”, their research provides a mechanism to explain these observations. The study’s authors commented that, “we propose that activating the lysosome/autophagy pathway, and its communication with mitochondria, represents a potential therapeutic target that could rejuvenate two key organelles that become impaired in the RPE of patients with dry AMD”.
While anti-VEGF treatments have been approved against neovascular AMD since 2006, the non-neovascular form, or dry-AMD, has only recently received approval in 2023, and therefore real-world data may require long-term outcomes in due course. To date, these more recent treatments on dry AMD appear to slow the pathology but do not prevent its progression, and therefore there is a considerable worldwide research drive to explore new targets aimed at developing new technologies.
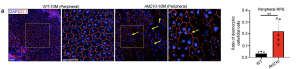
In the current research paper, an RPE-specific Akt2 knockin (KI) mouse showed that AKT2 upregulation was specific to the RPE and not present in the neurosensory retina and the Akt2 KI mice appeared to develop an AMD-like phenotype, beginning at 10 months with mild phenotypic worsening by 15 months old.
Figure – Akt2 upregulation triggers lysosomal dysfunction and an AMD-like phenotype in mice. [a] Quantification of ZO-1 immunostaining on RPE flatmounts from 10-month-old Akt2 KI mice shows an increased number of dysmorphic RPE cells in the peripheral regions (arrows), not seen in age-matched WT RPE. n (biological replicate)=5. Scale bar = 50 μm.
Open Access article is licensed under a Creative Commons Attribution 4.0 International License; Ghosh et al, The AKT2/SIRT5/TFEB pathway as a potential therapeutic target in nonneovascular AMD, Nature Communications, 2024 Jul 21;15(1):6150.
The overexpression of AKT2 was associated with the loss of lysosomal function and the animal models appeared to develop an-AMD degeneration. In addition, RPE cells from human donor cells with AMD carrying the CFH Y402H variants appeared to have a relatively greater expression of AKT2 and these showed defective lysosomal function and observations of drusen deposits.
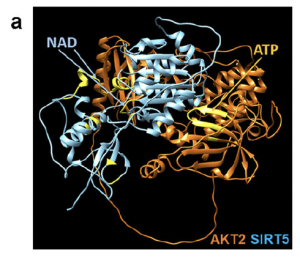
Following the work, the researchers then used computer modelling to show that AKT2 (orange) may bind directly to sirtuin 5 (SIRT5) (blue), indicating catalytic activity and these structural interactions may provide multiple opportunities from a therapeutical approach, pharmacologically, biologically and/or genetically.
Figure – AKT2/SIRT5 [sirtuin] signaling axis regulates TFEB (transcription factor EB/E3) activity. [a] A hypothetical heterodimer of human AKT2/SIRT5 suggests a potential protein–protein interaction between these proteins. The ribbon structures of AKT2 and SIRT5 are colored orange and cyan, respectively. Residues related to the AKT2 and SIRT5 active sites are shown in yellow. The model suggests that both proteins have active sites with potential exposure to substrates and ligands, indicating that the AKT2/SIRT5 complex is catalytically active.
Open Access article is licensed under a Creative Commons Attribution 4.0 International License; Ghosh et al, The AKT2/SIRT5/TFEB pathway as a potential therapeutic target in nonneovascular AMD, Nature Communications, 2024 Jul 21;15(1):6150.
In conclusion, the researchers stated that, “collectively, these findings suggest that targeting the AKT2/SIRT5/TFEB pathway may be an effective therapy to delay the progression of dry AMD”. Given the enormous unmet need on AMD globally, it is likely there will be increased interest on these opportunities for researchers, clinicians, industry and patients.