Researchers at the School of Pharmacy, University of Eastern Finland, and the Gavin Herbert Eye Institute-Center for Translational Vision Research, Department of Ophthalmology, University of California, Irvine, has published a new study using a combination of re-purposed drugs to treat retinal degenerations. The “disease-modifying therapies”(DMT), using a combination of tamsulosin, metoprolol and bromocriptine, has shown improved functional vision on retinopathy models in pre-clinical trials. The article, published in the journal Nature Communications (2024, 15:5943), provides an exciting approach for a potential disease treatment independent of a range of mutations that may arise from retinitis pigmentosa (RP). According to first-author of the paper, Dr. Henri Leinonen, Adjunct Professor of Neuropharmacology at the University of Eastern Finland, has commented that, “in drug repurposing, it does not matter to which diseases or conditions the drugs were originally developed for, but it is the molecular-level effects of drugs, or pharmacology, that count”.
Mutations in over 289 genes lead to retinal dysfunction, degeneration and sight loss to date. Each of these genes may carry multiple variants and to address such variants could require different types of treatments, dependent on the root cause and their pathophysiology. As outlined in their paper, “management of all those mutations by targeted therapies, mainly gene therapy, is likely to remain impractical and prohibitively expensive for the foreseeable future. Alternatively, a disease-modifying treatment (DMT), which aims to substantially diminish pathological mechanisms that drive cells to their demise regardless of the underlying etiology, could be more attainable for larger patient populations, and could benefit more patients faster. DMTs in development often utilize drug repurposing, which can significantly reduce drug development risks, timeline, and cost”.
In the current researchers’ study, the team utilized a systems pharmacology-based approach and targeted retina-expressing Gq-, Gs- and Gi-coupled GPCRs (G-protein coupled receptor) simultaneously, using the clinically approved drugs tamsulosin (T), metoprolol (M), and bromocriptine (B) – as prototype compounds, combined together as “TMB”. The strategy suppresses intracellular cAMP and Ca2+ activity via GPCR modulation using a coadministration approach. “TMB treatment” at low doses of each compound appears to prevent acute bright light-induced retinal degeneration (RD) in models of Stargardt disease, Oguchi disease, congenital stationary night blindness and in normal models. The researchers observed that “the required doses for a therapeutic effect against light-induced RD are much larger if the same drugs are administered individually as a monotherapy, suggesting therapeutic synergy by the TMB drug cocktail”.
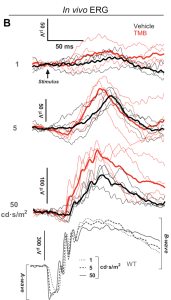
Fig 1(B): TMB administration stabilizes retinal transcriptome and improves visual function in Rpe65−/− mice. (B) Group-averaged scotopic ERGs. Thin lines represent responses from individual mice. (Open Access – this article is licensed under a Creative Commons Attribution 4.0 International License; Leinonen et al, Nature Communications, 2024, 15:5943, https://doi.org/10.1038/s41467-024-50033-5).
Scotopic ERG recordings after 1-month on treatment showed more robust light responses with the TMB-treated than with the vehicle-treated cyclic light-reared Rpe65-/- mice. The mean ( ± SD) b-wave amplitude in the vehicle-group was 142 ± 42 μV and in TMB-group 214 ± 28 μV in response to the highest intensity flash (50 cd·s/m2). While the study provides an early view on their approach, significant work will require further data on larger models, descriptive and inferential statistics and subsequent human pivotal trials however, given the considerable heterogeneity on RP suggests that the opportunity may be considerably attractive for clinicians and industry.
[Note: Tamsulosin, approved in 1997, is used to treat symptomatic benign prostatic hyperplasia (BPH) and chronic prostatitis (and passing of kidney stones); Metoprolol, approved in 1978, is used to treat high blood pressure, angina, and myocardial infarction and is on the World Health Organization’s List of Essential Medicines; Bromocriptine, approved in 1975, is used in the treatment of pituitary tumors, Parkinson’s disease, hyperprolactinaemia, neuroleptic malignant syndrome, and on type 2 diabetes].