Researchers based at the Nuffield Department of Clinical Neurosciences, The John Radcliffe Hospital, University of Oxford, have reported a new study on the mechanism of progressive chorioretinal atrophy, following administration of voretigene neparvovec (VN) (Luxturna, indicated by biallelic RPE-65-LCA). The study was conducted in non-human primates (NHP) to determine if atrophy was caused by the treatment itself (AAV-RPE65 gene therapy), the surgical procedure, or both. Following the analyses of the data, the researchers stated that, “atrophic changes after gene therapy with AAV-based vector systems are not primarily due to surgical trauma”, and that an adverse effect of chorioretinal atrophy (CRA) appears to increase with the dose given. This answer from the study provides a “highly relevant to the field looking to minimize adverse effects and maximize the benefit for our patients”, commented by a lead author of the research, Prof. M. Dominik Fischer, MD, PhD.
VN represents the first licensed treatment for patients with vision loss due to inherited retinal degenerations (IRD) caused by confirmed biallelic RPE65 mutations by both the FDA and EMA, now applied to hundreds of patients worldwide. The course of treatment comprises a single injection into each eye, understood with no further intervention required. The researchers at the University of Oxford have recently reported that more than 10% of patients treated with VN appear to present with CRA in the years following gene therapy. The key question posed by the Oxford group was, “[i]s it unique to treatment with VN and the RPE65- deficient retina, or is it a consequence of inherent immunogenic or otherwise toxic properties of the AAV vector system and/or its preparation, and therefore potentially not limited to VN?” In the current paper, the researchers evaluated subretinal injections as a single dose of either 1×1011 (low dose) or 1×1012 (high dose) vector genomes (vg) of high clinical grade rAAV2/8, designed to treat PDE6A-associated retinitis pigmentosa. The development of atrophic changes was monitored for 12 weeks and quantified using fundus autofluorescence (AF) imaging and using OCT imagery.
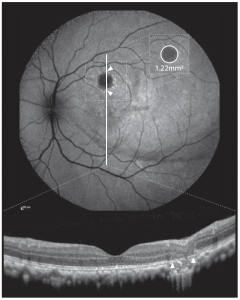
Figure 1. Atrophy at the injection site was quantified using fundus autofluorescence imaging (AF, top) and verified using OCT (bottom). Measurements were taken with the proprietary Heidelberg Engineering Eye Explorer (HEYEX 2). Sample measurement: 1.22 mm2. Areas of atrophy were verified using OCT (white arrows) to exclude false positive calls of atrophy in areas with confounding effects interfering with AF signal (e.g., pigment displacement e AF image: faint hypofluorescent arch along inferior bleb margin). This is an open access article under the CC BY license (http:// creativecommons.org/licenses/by/4.0/), authored by Seitz et al, entitled, “Dose-Dependent Progression of Chorioretinal Atrophy at the Injection Site After Subretinal Injection of rAAV2/8 in Nonhuman Primates”, published in Ophthalmology Science Volume 4, Number 5, October 2024.
The results of the study showed progressive atrophy at the injection site developed in 54% of high-dose-treated, 27% of low-dose treated, and 0% of sham-treated eyes. At the end of observation, the mean ± (SD) area of atrophy in AF was 1.19 ± 1.75 mm2 , 0.25 ± 0.50 mm2 , and 0.0 ± 0.0 mm2 , respectively (sham high dose: P ¼ 0.01). Atrophic lesions in AF (P ¼ 0.01) and fluorescence angiography (P ¼ 0.02) were significantly larger in high-dose-treated eyes, compared with sham-treated eyes. In addition, the rate of progression in high-dose-treated eyes was 4.1 higher compared with low-dose-treated eyes. In conclusion, subretinal injection of AAV induced dose-dependent, progressive retinal atrophy at the site of injection and the research team commented that, “our results suggest that inherent properties of the AAV vector platform can likely cause or aggravate the development of progressive chorioretinal atrophy. The fact that this is not observed in the sham group rules out surgical trauma alone as its main cause. The dose-dependent nature further highlights a causal relationship with AAV and/or its preparation. The fact that this was observed with a clinical grade AAV of a different pseudotype (AAV8) to that used for VN (AAV2) strengthens the notion that the issue of CRA may not be limited to VN”.