Researchers based at the MRC Human Genetics Unit, at the University of Edinburgh, and the Western General Hospital (Edinburgh), have reported a new study indicating that disc formation, at the outer segments of the photoreceptors, appears to be an actin-driven process, rather than a passive process or blebbing. Disc shedding on the outers segments may arise from alterations of the RPGR protein, and localisation of cofilin, an actin-binding protein acting in a central role in cell motility, contraction and transcription regulation. Research results suggest that RPGR proteins may compromise cofilin activity, and the perturbation of disc shedding results in outer segment abnormalities, retinal stress, photoreceptor degeneration and loss of vision. While the research is at an early stage, the outcomes are significant in their report and modulation of these actin manipulations, in due course, could be targeted therapeutically.
Variants of the retinitis pigmentosa GTPase regulator (RPGR) may lead to X-linked retinitis pigmentosa (XLRP) and the majority of mutations in the gene are found in exon open reading frame 15 (ORF15). It gene encodes a ciliary protein that regulates trafficking of proteins to the outer segment of photoreceptors. RP affects an estimate of 1 in 3,000 people world-wide, arising from 289 genes to date, while alterations of the RPGR protein may account for 70–90% of X-linked RP (XLRP) and 10-15% of all RP resulting in a severe form of the disease. The current research report, published in Nature Communications (2024 May 21;15(1):4316), used cryo-electron tomography (cryoET) and immunohistochemistry showing that cofilin localisation to photoreceptor connecting cilium (CC) were in normal retinas, and partially lost in mutated models not seen in cofilin in knockout mice. Co-immunoprecipitation experiments using retinal lysates confirmed endogenous cofilin and the retinal specific isoform of RPGR occur in complex in the retina.
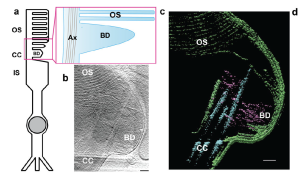
Figure. 1 Photoreceptor disc formation is an actin-driven process. (a) Schematic of a rod photoreceptor depicts the cell’s inner segment (IS) with the highly modified connecting cilium (CC) extending from its apex, containing a microtubule-based axoneme (Ax). From here an expanse of folded membrane extends, forming the outer segment (OS) discs. Basal discs (BD) are continually added at its base. (b) A projection from a tomogram of the basal disc of a wild-type photoreceptor. (c) A slice through a tomographic reconstruction, segmented to highlight the ciliary and disc membranes (green), microtubule-based axoneme (cyan) and microfilaments (purple). Microfilaments extend from the connecting cilium into the basal disc. [Open Access article, under a Creative Commons Attribution 4.0 International License (visit http://creativecommons.org/licenses/by/4.0/) , Megaw et al, Nat Commun. 2024 May 21;15(1):4316. doi: 10.1038/s41467-024-48639-w].
The Scottish team outlined that cofilin is known to regulate primary cilia length through actin rearrangement and the observation that “cofilin is present in aborted photoreceptor ectosomes when normal disc formation is disrupted further supports this model”. In addition, in their report, the authors stated that, “[n]o treatment currently exists for any form of RP. Whilst gene therapy offers hope, long-term data for the only FDA- and EMA approved gene therapy drug for genetic eye disease is less encouraging. Complementary approaches must therefore be sought. Here, we show that pharmaco-manipulation of actin activity could reverse the underlying cellular phenotypes disrupting disc biogenesis. Further work is required to address the translational potential of our study, specifically to establish if long-term cofilin modulation could slow the retinal degeneration seen in RPGR/XLRP patients”.